Effect of rituximab on human in vivo antibody immune responses
- PMID: 21908031
- PMCID: PMC3659395
- DOI: 10.1016/j.jaci.2011.08.008
Effect of rituximab on human in vivo antibody immune responses
Abstract
Background: B-lymphocyte depletion with rituximab has been shown to benefit patients with various autoimmune diseases. We have previously demonstrated that this benefit is also apparent in patients with newly diagnosed type 1 diabetes.
Objectives: The effect of rituximab on in vivo antibody responses, particularly during the period of B-lymphocyte depletion, is incompletely determined. This study was designed to assess this knowledge void.
Methods: In patients with recent-onset type 1 diabetes treated with rituximab (n = 46) or placebo (n = 29), antibody responses to neoantigen phiX174 during B-lymphocyte depletion and with hepatitis A (as a second neoantigen) and tetanus/diphtheria (as recall antigens) after B-lymphocyte recovery were studied. Anti- tetanus, diphtheria, mumps, measles, and rubella titers were measured before and after treatment by means of ELISA. Antibody titers and percentage IgM versus percentage IgG to phiX174 were measured by means of phage neutralization. B-lymphocyte subsets were determined by means of flow cytometry.
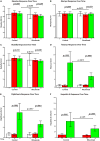
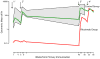
Results: No change occurred in preexisting antibody titers. Tetanus/diphtheria and hepatitis A immunization responses were protective in the rituximab-treated subjects, although significantly blunted compared with those seen in the controls subjects, when immunized at the time of B-lymphocyte recovery. Anti-phiX174 responses were severely reduced during the period of B-lymphocyte depletion, but with B-lymphocyte recovery, anti-phiX174 responses were within the normal range.
Conclusions: During the time of B-lymphocyte depletion, rituximab recipients had a decreased antibody response to neoantigens and significantly lower titers after recall immunization with diphtheria and tetanus toxoid. With recovery, immune responses return toward normal. Immunization during the time of B-lymphocyte depletion, although ineffective, does not preclude a subsequent response to the antigen.
Copyright © 2011 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: T R. Torgerson has consultant arrangements with Baxter Biosciences. J. M. Lachin has consultant arrangements with Genentech, Bayhill Therapeutics, GlaxoSmithKline, and TolerRx. C. Greenbaum receives research support from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, the Juvenile Diabetes Research Foundation International, and the National Institutes of Health/National Institute of Allergy and Infectious Diseases. J. S. Skyler is on the Board of Directors for Amylin Pharmaceuticals and DexCom Inc, has consultant arrangements with SanofiAventis and BD Technologies, and has received research support from Bayhill Therapeutics, Halozyme Inc, and Osiris Therapeutics. The rest of the authors have declared that they have no conflict of interest.
Figures
Similar articles
-
Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial.Arthritis Rheum. 2010 Jan;62(1):64-74. doi: 10.1002/art.25034. Arthritis Rheum. 2010. PMID: 20039397 Clinical Trial.
-
Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage phiX174.Am J Transplant. 2005 Jan;5(1):50-7. doi: 10.1111/j.1600-6143.2003.00646.x. Am J Transplant. 2005. PMID: 15636611
-
Rituximab, B-lymphocyte depletion, and preservation of beta-cell function.N Engl J Med. 2009 Nov 26;361(22):2143-52. doi: 10.1056/NEJMoa0904452. N Engl J Med. 2009. PMID: 19940299 Free PMC article. Clinical Trial.
-
[Progress in B-cell targeting therapy for the treatment of systemic lupus erythematosus].J UOEH. 2011 Jun 1;33(2):173-81. doi: 10.7888/juoeh.33.173. J UOEH. 2011. PMID: 21702122 Review. Japanese.
-
B cells as therapeutic targets for rheumatic diseases.Curr Opin Rheumatol. 2004 May;16(3):180-5. doi: 10.1097/00002281-200405000-00003. Curr Opin Rheumatol. 2004. PMID: 15103242 Review.
Cited by
-
Immune therapy in type 1 diabetes mellitus.Nat Rev Endocrinol. 2013 Feb;9(2):92-103. doi: 10.1038/nrendo.2012.237. Epub 2013 Jan 8. Nat Rev Endocrinol. 2013. PMID: 23296174 Review.
-
Acute hepatitis B virus infection with delayed appearance of hepatitis B core antibody in an immunocompromised patient: a case report.J Med Case Rep. 2017 Apr 17;11(1):111. doi: 10.1186/s13256-017-1264-9. J Med Case Rep. 2017. PMID: 28412974 Free PMC article.
-
Modulation of Multiple Sclerosis and Its Animal Model Experimental Autoimmune Encephalomyelitis by Food and Gut Microbiota.Front Immunol. 2017 Sep 5;8:1081. doi: 10.3389/fimmu.2017.01081. eCollection 2017. Front Immunol. 2017. PMID: 28928747 Free PMC article. Review.
-
Hematopoietic Cell Transplantation for Systemic Sclerosis-A Review.Cells. 2022 Dec 3;11(23):3912. doi: 10.3390/cells11233912. Cells. 2022. PMID: 36497169 Free PMC article. Review.
-
Rituximab-treated patients have a poor response to influenza vaccination.J Clin Immunol. 2013 Feb;33(2):388-96. doi: 10.1007/s10875-012-9813-x. Epub 2012 Oct 14. J Clin Immunol. 2013. PMID: 23064976 Free PMC article.
References
-
- Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–32. - PubMed
-
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. - PubMed
-
- Tobinai K, Kobayashi Y, Narabayashi M, Ogura M, Kagami Y, Morishima Y, et al. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B-cell lymphoma The IDEC-C2B8 Study Group. Ann Oncol. 1998;9:527–34. - PubMed
-
- Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

