Induction of DNA damage signaling by oxidative stress in relation to DNA replication as detected using "click chemistry"
- PMID: 21905210
- PMCID: PMC3238684
- DOI: 10.1002/cyto.a.21137
Induction of DNA damage signaling by oxidative stress in relation to DNA replication as detected using "click chemistry"
Abstract
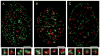
Induction of DNA damage by oxidants such as H(2) O(2) activates the complex network of DNA damage response (DDR) pathways present in cells to initiate DNA repair, halt cell cycle progression, and prepare an apoptotic reaction. We have previously reported that activation of Ataxia Telangiectasia Mutated protein kinase (ATM) and induction of γH2AX are among the early events of the DDR induced by exposure of cells to H(2) O(2) , and in human pulmonary carcinoma A549 cells, both events were expressed predominantly during S-phase. This study was designed to further explore a correlation between these events and DNA replication. Toward this end, we utilized 5-ethynyl-2'deoxyuridine (EdU) and the "click chemistry" approach to label DNA during replication, followed by exposure of A549 cells to H(2) O(2) . Multiparameter laser scanning cytometric analysis of these cells made it possible to identify DNA replicating cells and directly correlate H(2) O(2) -induced ATM activation and induction of γH2AX with DNA replication on a cell by cell basis. After pulse-labeling with EdU and exposure to H(2) O(2) , confocal microscopy was also used to examine the localization of DNA replication sites ("replication factories") versus the H2AX phosphorylation sites (γH2AX foci) in nuclear chromatin in an attempt to observe the absence or presence of colocalization. The data indicate a close association between DNA replication and H2AX phosphorylation in A549 cells, suggesting that these DNA damage response events may be triggered by stalled replication forks and perhaps also by induction of DNA double-strand breaks at the primary DNA lesions induced by H(2) O(2) .
Copyright © 2011 International Society for Advancement of Cytometry.
Figures
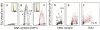


Similar articles
-
Kinetics of the UV-induced DNA damage response in relation to cell cycle phase. Correlation with DNA replication.Cytometry A. 2010 Mar;77(3):285-93. doi: 10.1002/cyto.a.20839. Cytometry A. 2010. PMID: 20014310 Free PMC article.
-
DNA damage response induced by tobacco smoke in normal human bronchial epithelial and A549 pulmonary adenocarcinoma cells assessed by laser scanning cytometry.Cytometry A. 2009 Oct;75(10):840-7. doi: 10.1002/cyto.a.20778. Cytometry A. 2009. PMID: 19658174 Free PMC article.
-
DNA damage signaling, impairment of cell cycle progression, and apoptosis triggered by 5-ethynyl-2'-deoxyuridine incorporated into DNA.Cytometry A. 2013 Nov;83(11):979-88. doi: 10.1002/cyto.a.22396. Epub 2013 Sep 30. Cytometry A. 2013. PMID: 24115313 Free PMC article.
-
Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents.Cytometry A. 2007 Sep;71(9):648-61. doi: 10.1002/cyto.a.20426. Cytometry A. 2007. PMID: 17622968 Free PMC article. Review.
-
Analysis of individual molecular events of DNA damage response by flow- and image-assisted cytometry.Methods Cell Biol. 2011;103:115-47. doi: 10.1016/B978-0-12-385493-3.00006-1. Methods Cell Biol. 2011. PMID: 21722802 Free PMC article. Review.
Cited by
-
SC-III3, a novel scopoletin derivative, induces cytotoxicity in hepatocellular cancer cells through oxidative DNA damage and ataxia telangiectasia-mutated nuclear protein kinase activation.BMC Cancer. 2014 Dec 19;14:987. doi: 10.1186/1471-2407-14-987. BMC Cancer. 2014. PMID: 25527123 Free PMC article.
-
The role of oxidative stress in 63 T-induced cytotoxicity against human lung cancer and normal lung fibroblast cell lines.Invest New Drugs. 2019 Oct;37(5):849-864. doi: 10.1007/s10637-018-0704-8. Epub 2018 Nov 29. Invest New Drugs. 2019. PMID: 30498945 Free PMC article.
-
Attenuation of constitutive DNA damage signaling by 1,25-dihydroxyvitamin D3.Aging (Albany NY). 2012 Apr;4(4):270-8. doi: 10.18632/aging.100450. Aging (Albany NY). 2012. PMID: 22498490 Free PMC article.
-
New insights into cell cycle and DNA damage response machineries through high-resolution AMICO quantitative imaging cytometry.Cell Prolif. 2013 Oct;46(5):497-500. doi: 10.1111/cpr.12050. Epub 2013 Aug 17. Cell Prolif. 2013. PMID: 23952744 Free PMC article.
-
Adult Cardiomyocyte Cell Cycle Detour: Off-ramp to Quiescent Destinations.Trends Endocrinol Metab. 2019 Aug;30(8):557-567. doi: 10.1016/j.tem.2019.05.006. Epub 2019 Jun 28. Trends Endocrinol Metab. 2019. PMID: 31262545 Free PMC article. Review.
References
-
- Beckman BK, Ames BN. The free radical theory of aging matures. Phys Rev. 1998;78:547–581. - PubMed
-
- Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:13300–13305. - PubMed
-
- Gorbunova V, Seluanov A. Making ends meet in old age: DSB repair and aging. Mech Ageing Dev. 2005;126:621–628. - PubMed
-
- Karanjawala ZE, Lieber MR. DNA damage and aging. Mech Ageing Dev. 2004;125:405–416. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

