Imaging heart development using high-resolution episcopic microscopy
- PMID: 21893408
- PMCID: PMC3368266
- DOI: 10.1016/j.gde.2011.07.004
Imaging heart development using high-resolution episcopic microscopy
Abstract
Development of the heart in vertebrate embryos is a complex process in which the organ is continually remodelled as chambers are formed, valves sculpted and connections established to the developing vascular system. Investigating the genetic programmes driving these changes and the environmental factors that may influence them is critical for our understanding of congenital heart disease. A recurrent challenge in this work is how to integrate studies as diverse as those of cardiac gene function and regulation with an appreciation of the localised interactions between cardiac tissues not to mention the manner in which both may be affected by cardiac function itself. Meeting this challenge requires an accurate way to analyse the changes in 3D morphology of the developing heart, which can be swift or protracted and both dramatic or subtle in consequence. Here we review the use of high-resolution episcopic microscopy as a simple and effective means to examine organ structure and one that allows modern computing methods pioneered by clinical imaging to be applied to the embryonic heart.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
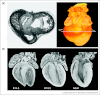

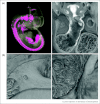
Similar articles
-
Quantification of the detailed cardiac left ventricular trabecular morphogenesis in the mouse embryo.Med Image Anal. 2018 Oct;49:89-104. doi: 10.1016/j.media.2018.08.001. Epub 2018 Aug 2. Med Image Anal. 2018. PMID: 30114550
-
Three-dimensional analysis of molecular signals with episcopic imaging techniques.Methods Mol Biol. 2007;411:35-46. doi: 10.1007/978-1-59745-549-7_4. Methods Mol Biol. 2007. PMID: 18287637
-
Molecular imaging of the embryonic heart: Fables and facts on 3D imaging of gene expression patterns.Birth Defects Res C Embryo Today. 2004 Sep;72(3):224-40. doi: 10.1002/bdrc.20018. Birth Defects Res C Embryo Today. 2004. PMID: 15495186 Review.
-
Morphogenesis of myocardial trabeculae in the mouse embryo.J Anat. 2016 Aug;229(2):314-25. doi: 10.1111/joa.12465. Epub 2016 Mar 29. J Anat. 2016. PMID: 27020702 Free PMC article.
-
3D Anatomy of the Developing Heart: Understanding Ventricular Septation.Cold Spring Harb Perspect Biol. 2020 Nov 2;12(11):a037465. doi: 10.1101/cshperspect.a037465. Cold Spring Harb Perspect Biol. 2020. PMID: 31988142 Free PMC article. Review.
Cited by
-
High-contrast X-ray micro-radiography and micro-CT of ex-vivo soft tissue murine organs utilizing ethanol fixation and large area photon-counting detector.Sci Rep. 2016 Jul 27;6:30385. doi: 10.1038/srep30385. Sci Rep. 2016. PMID: 27461900 Free PMC article.
-
Phenotyping structural abnormalities in mouse embryos using high-resolution episcopic microscopy.Dis Model Mech. 2014 Oct;7(10):1143-52. doi: 10.1242/dmm.016337. Dis Model Mech. 2014. PMID: 25256713 Free PMC article.
-
Fractal analysis in cardiovascular magnetic resonance: prognostic value of biventricular trabecular complexity in hypertrophic cardiomyopathy.Cardiovasc Diagn Ther. 2023 Dec 15;13(6):1030-1042. doi: 10.21037/cdt-23-162. Epub 2023 Oct 26. Cardiovasc Diagn Ther. 2023. PMID: 38162100 Free PMC article.
-
Development and Morphology of the Ventricular Outflow Tracts.World J Pediatr Congenit Heart Surg. 2016 Sep;7(5):561-77. doi: 10.1177/2150135116651114. World J Pediatr Congenit Heart Surg. 2016. PMID: 27587491 Free PMC article. Review.
-
Visualising the Cardiovascular System of Embryos of Biomedical Model Organisms with High Resolution Episcopic Microscopy (HREM).J Cardiovasc Dev Dis. 2018 Dec 15;5(4):58. doi: 10.3390/jcdd5040058. J Cardiovasc Dev Dis. 2018. PMID: 30558275 Free PMC article. Review.
References
-
- Born G. Die Plattenmodelliermethode. Arch Mikr Anat. 1883;22:584–599.
-
- His W . FCW Vogel; Leipzig: 1885. Anatomie menschlicher Embryonen. III. Zur Geschichte der Organe.
-
- His W. Über die Methoden der plastischen Rekonstruktion und über deren Bedeutung für Anatomie und Entwicklungsgeschichte. Anatomischer Anzeiger. 1887;2:382–394.
-
- Braverman M.S., Braverman I.M. Three-dimensional reconstructions of objects from serial sections using a microcomputer graphics system. J Invest Dermatol. 1986;86:290–294. - PubMed
-
- McLean M.R., Prothero J. Coordinated three-dimensional reconstruction from serial sections at macroscopic and microscopic levels of resolution: the human heart. Anat Rec. 1987;219:379–434. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

