Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain
- PMID: 21880915
- PMCID: PMC6703268
- DOI: 10.1523/JNEUROSCI.2840-11.2011
Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain
Abstract
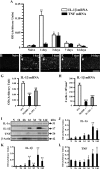
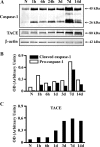
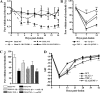
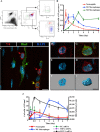
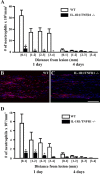
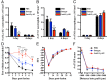
IL-1β and TNF are potential targets in the management of neuropathic pain after injury. However, the importance of the IL-1 and TNF systems for peripheral nerve regeneration and the mechanisms by which these cytokines mediate effects are to be fully elucidated. Here, we demonstrate that mRNA and protein levels of IL-1β and TNF are rapidly upregulated in the injured mouse sciatic nerve. Mice lacking both IL-1β and TNF, or both IL-1 type 1 receptor (IL-1R1) and TNF type 1 receptor (TNFR1), showed reduced nociceptive sensitivity (mechanical allodynia) compared with wild-type littermates after injury. Microinjecting recombinant IL-1β or TNF at the site of sciatic nerve injury in IL-1β- and TNF-knock-out mice restored mechanical pain thresholds back to levels observed in injured wild-type mice. Importantly, recovery of sciatic nerve function was impaired in IL-1β-, TNF-, and IL-1β/TNF-knock-out mice. Notably, the infiltration of neutrophils was almost completely prevented in the sciatic nerve distal stump of mice lacking both IL-1R1 and TNFR1. Systemic treatment of mice with an anti-Ly6G antibody to deplete neutrophils, cells that play an essential role in the genesis of neuropathic pain, did not affect recovery of neurological function and peripheral axon regeneration. Together, these results suggest that targeting specific IL-1β/TNF-dependent responses, such as neutrophil infiltration, is a better therapeutic strategy for treatment of neuropathic pain after peripheral nerve injury than complete blockage of cytokine production.
Figures
Similar articles
-
Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice.J Pain. 2011 Mar;12(3):370-83. doi: 10.1016/j.jpain.2010.08.003. J Pain. 2011. PMID: 20889388
-
Lack of interleukin-17 leads to a modulated micro-environment and amelioration of mechanical hypersensitivity after peripheral nerve injury in mice.Pain. 2014 Jul;155(7):1293-1302. doi: 10.1016/j.pain.2014.04.004. Epub 2014 Apr 8. Pain. 2014. PMID: 24721689
-
Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents.Mol Pain. 2016 May 12;12:1744806916646784. doi: 10.1177/1744806916646784. Print 2016. Mol Pain. 2016. PMID: 27175012 Free PMC article.
-
Hyperbaric oxygenation therapy alleviates chronic constrictive injury-induced neuropathic pain and reduces tumor necrosis factor-alpha production.Anesth Analg. 2011 Sep;113(3):626-33. doi: 10.1213/ANE.0b013e31821f9544. Epub 2011 May 19. Anesth Analg. 2011. PMID: 21596875
-
Tumor Necrosis Factor and Interleukin Modulators for Pathologic Pain States: A Narrative Review.Pain Ther. 2024 Jun;13(3):481-493. doi: 10.1007/s40122-024-00603-8. Epub 2024 May 9. Pain Ther. 2024. PMID: 38724743 Free PMC article. Review.
Cited by
-
Update on Biomarkers of Chronic Inflammatory Processes Underlying Diabetic Neuropathy.Int J Mol Sci. 2024 Sep 27;25(19):10395. doi: 10.3390/ijms251910395. Int J Mol Sci. 2024. PMID: 39408723 Free PMC article. Review.
-
Pathway-specific TNF-mediated metaplasticity in hippocampal area CA1.Sci Rep. 2022 Feb 2;12(1):1746. doi: 10.1038/s41598-022-05844-1. Sci Rep. 2022. PMID: 35110639 Free PMC article.
-
The impact of obesity and smoking on young individuals suffering from lumbar disc herniation: a retrospective analysis of 97 cases.Neurosurg Rev. 2020 Oct;43(5):1297-1303. doi: 10.1007/s10143-019-01151-y. Epub 2019 Aug 14. Neurosurg Rev. 2020. PMID: 31414196 Free PMC article.
-
Profiling the proteomic inflammatory state of human astrocytes using DIA mass spectrometry.J Neuroinflammation. 2018 Nov 30;15(1):331. doi: 10.1186/s12974-018-1371-6. J Neuroinflammation. 2018. PMID: 30501627 Free PMC article.
-
The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration.J Neuroinflammation. 2022 Jul 12;19(1):179. doi: 10.1186/s12974-022-02497-9. J Neuroinflammation. 2022. PMID: 35820932 Free PMC article.
References
-
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. - PubMed
-
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
