Pharmacokinetics and pharmacodynamics of oral and transdermal 17β estradiol in girls with Turner syndrome
- PMID: 21880799
- PMCID: PMC3205885
- DOI: 10.1210/jc.2011-1449
Pharmacokinetics and pharmacodynamics of oral and transdermal 17β estradiol in girls with Turner syndrome
Abstract
Context: The type, dose, and route of 17β-estradiol (E(2)) used to feminize girls with Turner syndrome (TS) is not well established.
Objective: The objective of the study was to characterize pharmacokinetics and pharmacodynamics of oral vs. transdermal E(2).
Setting: The study was conducted at a clinical research center.
Subjects: Ten girls with TS, mean age 17.7 ± 0.4 (se) yr and 20 normally menstruating controls (aged 16.8 ± 0.4 yr) participated in the study.
Interventions: TS subjects were randomized 2 wk each to: low-dose daily oral (0.5 mg) and biweekly transdermal E(2) (0.0375 mg) with 2 wk washout in between or high-dose oral (2.0 mg) and transdermal (0.075 mg), studied for 24 h each. Tandem mass spectrometry E(2) and estrone (E(1)) assays and a recombinant cell bioassay were used.
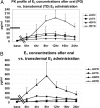
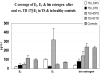
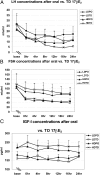
Results: Controls consisted of the following: E(2), 96 ± 11 pg/ml (se), E(1), 70 ± 7 (mean follicular/luteal). TS consisted of the following: E(2), average concentration on low-dose oral, 18 ± 2.1 pg/ml, low-dose transdermal, 38 ± 13, high-dose oral, 46 ± 15, high-dose transdermal, 114 ± 31 pg/ml. E(1) concentrations were much higher on oral E(2) (low or high dose) than transdermal in TS and higher than controls. Bioestrogen was closest to normal in the high-dose transdermal group. LH and FSH decreased more in transdermal than oral low-dose routes and similarly in the high-dose oral and transdermal groups. IGF-I concentrations were variable (P = NS) among groups, and low-density lipoprotein/high-density lipoprotein cholesterol responses were variable.
Conclusions: Transdermal E(2) results in E(2), E(1), and bioestrogen concentrations closer to normal and achieves greater suppression of LH/FSH in lower doses compared with normal. Whether the long-term metabolic effects of estrogen differ using the same form of E(2), depending on route, awaits further study in TS.
Figures
Similar articles
-
Metabolic effects of oral versus transdermal 17β-estradiol (E₂): a randomized clinical trial in girls with Turner syndrome.J Clin Endocrinol Metab. 2013 Jul;98(7):2716-24. doi: 10.1210/jc.2012-4243. Epub 2013 May 15. J Clin Endocrinol Metab. 2013. PMID: 23678038 Free PMC article. Clinical Trial.
-
Estrogen therapy in Turner syndrome: does the type, dose and mode of delivery matter?Pediatr Endocrinol Rev. 2012 May;9 Suppl 2:718-22. Pediatr Endocrinol Rev. 2012. PMID: 22946283 Review.
-
Is There a Difference between Ultrasonographic (US) Uterine Changes of Oral Versus Transdermal (TD) 17β Estradiol (17β E2) in Girls with Turner Syndrome (TS)? Own Experience and Literature Review.Pediatr Endocrinol Rev. 2018 Sep;16(1):178-185. doi: 10.17458/per.vol16.2018.kue.uschangesversustd. Pediatr Endocrinol Rev. 2018. PMID: 30371036
-
Impact of route of administration on genotoxic oestrogens concentrations using oral vs transdermal oestradiol in girls with Turner syndrome.Clin Endocrinol (Oxf). 2019 Jan;90(1):155-161. doi: 10.1111/cen.13869. Epub 2018 Oct 25. Clin Endocrinol (Oxf). 2019. PMID: 30281805 Clinical Trial.
-
Optimal Pubertal Induction in Girls with Turner Syndrome Using Either Oral or Transdermal Estradiol: A Proposed Modern Strategy.Horm Res Paediatr. 2019;91(3):153-163. doi: 10.1159/000500050. Epub 2019 Jun 5. Horm Res Paediatr. 2019. PMID: 31167218 Review.
Cited by
-
Hypogonadism in adolescent girls: treatment and long-term effects.Acta Biomed. 2022 Oct 26;93(5):e2022317. doi: 10.23750/abm.v93i5.13719. Acta Biomed. 2022. PMID: 36300209 Free PMC article.
-
The Care of Adolescents and Young Adults with Turner Syndrome: A Pediatric and Adolescent Gynecology Perspective.J Pediatr Adolesc Gynecol. 2022 Aug;35(4):429-434. doi: 10.1016/j.jpag.2022.02.002. Epub 2022 Mar 8. J Pediatr Adolesc Gynecol. 2022. PMID: 35272055 Free PMC article. Review.
-
Sex hormone replacement in Turner syndrome.Endocrine. 2012 Apr;41(2):200-19. doi: 10.1007/s12020-011-9569-8. Epub 2011 Dec 7. Endocrine. 2012. PMID: 22147393 Review.
-
Topically applied phospho-sulindac hydrogel is efficacious and safe in the treatment of experimental arthritis in rats.Pharm Res. 2013 Jun;30(6):1471-82. doi: 10.1007/s11095-012-0953-8. Epub 2013 Mar 13. Pharm Res. 2013. PMID: 23483440 Free PMC article.
-
Primary ovarian insufficiency: update on clinical and genetic findings.Front Endocrinol (Lausanne). 2024 Sep 26;15:1464803. doi: 10.3389/fendo.2024.1464803. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39391877 Free PMC article. Review.
References
-
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. 2002. Production and actions of estrogens. N Engl J Med 346:340–352 - PubMed
-
- Ross JL, Cassorla FG, Skerda MC, Valk IM, Loriaux DL, Cutler GB., Jr 1983. A preliminary study of the effect of estrogen dose on growth in Turner syndrome. N Engl J Med 309:1104–1106 - PubMed
-
- Mauras N, Rogol AD, Veldhuis JD. 1990. Increased hGH production rate after low-dose estrogen therapy in prepubertal girls with Turner syndrome. Pediatr Res 28:626–630 - PubMed
-
- Drobac S, Rubin K, Rogol AD, Rosenfield RL. 2006. A workshop on pubertal hormone replacement options in the United States. J Pediatr Endocrinol Metab 19:55–64 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

