The in vivo mechanism of action of CD20 monoclonal antibodies depends on local tumor burden
- PMID: 21880632
- PMCID: PMC3232265
- DOI: 10.3324/haematol.2011.047159
The in vivo mechanism of action of CD20 monoclonal antibodies depends on local tumor burden
Abstract
Background: CD20 monoclonal antibodies are widely used in clinical practice. Antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity and direct cell death have been suggested to be important effector functions for CD20 antibodies. However, their specific contributions to the in vivo mechanism of action of CD20 immunotherapy have not been well defined.
Design and methods: Here we studied the in vivo mechanism of action of type I (rituximab and ofatumumab) and type II (HuMab-11B8) CD20 antibodies in a peritoneal, syngeneic, mouse model with EL4-CD20 cells using low and high tumor burden.
Results: Interestingly, we observed striking differences in the in vivo mechanism of action of CD20 antibodies dependent on tumor load. In conditions of low tumor burden, complement was sufficient for tumor killing both for type I and type II CD20 antibodies. In contrast, in conditions of high tumor burden, activating FcγR (specifically FcγRIII), active complement and complement receptor 3 were all essential for tumor killing. Our data suggest that complement-enhanced antibody-dependent cellular cytotoxicity may critically affect tumor killing by CD20 antibodies in vivo. The type II CD20 antibody 11B8, which is a poor inducer of complement activation, was ineffective against high tumor burden.
Conclusions: Tumor burden affects the in vivo mechanism of action of CD20 antibodies. Low tumor load can be eliminated by complement alone, whereas elimination of high tumor load requires multiple effector mechanisms.
Figures

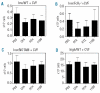
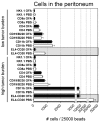
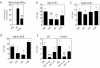

Similar articles
-
γδ T-cell killing of primary follicular lymphoma cells is dramatically potentiated by GA101, a type II glycoengineered anti-CD20 monoclonal antibody.Haematologica. 2011 Mar;96(3):400-7. doi: 10.3324/haematol.2010.029520. Epub 2010 Nov 25. Haematologica. 2011. PMID: 21109686 Free PMC article.
-
HuMab-7D8, a monoclonal antibody directed against the membrane-proximal small loop epitope of CD20 can effectively eliminate CD20 low expressing tumor cells that resist rituximab-mediated lysis.Haematologica. 2010 Dec;95(12):2063-71. doi: 10.3324/haematol.2010.025783. Epub 2010 Sep 17. Haematologica. 2010. PMID: 20851867 Free PMC article.
-
Engineered anti-CD20 antibodies with enhanced complement-activating capacity mediate potent anti-lymphoma activity.Cancer Sci. 2009 Dec;100(12):2411-8. doi: 10.1111/j.1349-7006.2009.01327.x. Epub 2009 Aug 25. Cancer Sci. 2009. PMID: 19758394 Free PMC article.
-
New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies.BioDrugs. 2011 Feb 1;25(1):13-25. doi: 10.2165/11539590-000000000-00000. BioDrugs. 2011. PMID: 21090841 Review.
-
The mechanisms of action of rituximab in the elimination of tumor cells.Semin Oncol. 2003 Feb;30(1 Suppl 2):3-8. doi: 10.1053/sonc.2003.50025. Semin Oncol. 2003. PMID: 12652458 Review.
Cited by
-
Improving Antibody Therapeutics by Manipulating the Fc Domain: Immunological and Structural Considerations.Annu Rev Biomed Eng. 2022 Jun 6;24:249-274. doi: 10.1146/annurev-bioeng-082721-024500. Epub 2022 Apr 1. Annu Rev Biomed Eng. 2022. PMID: 35363537 Free PMC article. Review.
-
A novel anti-CD19 monoclonal antibody (GBR 401) with high killing activity against B cell malignancies.J Hematol Oncol. 2014 Apr 14;7:33. doi: 10.1186/1756-8722-7-33. J Hematol Oncol. 2014. PMID: 24731302 Free PMC article.
-
Development of a Bispecific IgG1 Antibody Targeting BCMA and PDL1.Antibodies (Basel). 2024 Feb 20;13(1):15. doi: 10.3390/antib13010015. Antibodies (Basel). 2024. PMID: 38390876 Free PMC article.
-
Monitoring of the Complement System Status in Patients With B-Cell Malignancies Treated With Rituximab.Front Immunol. 2020 Nov 19;11:584509. doi: 10.3389/fimmu.2020.584509. eCollection 2020. Front Immunol. 2020. PMID: 33329558 Free PMC article.
-
A rationally-engineered IL-2 improves the antitumor effect of anti-CD20 therapy.Oncoimmunology. 2020 Jun 2;9(1):1770565. doi: 10.1080/2162402X.2020.1770565. Oncoimmunology. 2020. PMID: 32923126 Free PMC article.
References
-
- Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6(5):394–403. - PubMed
-
- Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44(16):3823–37. - PubMed
-
- Teeling JL, French RR, Cragg MS, van den BJ, Pluyter M, Huang H, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104(6):1793–800. - PubMed
-
- Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

