The role of mammalian sirtuins in the regulation of metabolism, aging, and longevity
- PMID: 21879449
- PMCID: PMC3745303
- DOI: 10.1007/978-3-642-21631-2_7
The role of mammalian sirtuins in the regulation of metabolism, aging, and longevity
Abstract
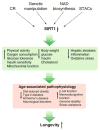
Ever since the discovery of sirtuins a decade ago, interest in this family of NAD-dependent deacetylases has exploded, generating multiple lines of evidence implicating sirtuins as evolutionarily conserved regulators of lifespan. In mammals, it has been established that sirtuins regulate physiological responses to metabolism and stress, two key factors that affect the process of aging. Further investigation into the intimate connection among sirtuins, metabolism, and aging has implicated the activation of SIRT1 as both preventative and therapeutic measures against multiple age-associated disorders including type 2 diabetes and Alzheimer's disease. SIRT1 activation has clear potential to not only prevent age-associated diseases but also to extend healthspan and perhaps lifespan. Sirtuin activating compounds and NAD intermediates are two promising ways to achieve these elusive goals.
Figures

Similar articles
-
Sirtuins, a promising target in slowing down the ageing process.Biogerontology. 2017 Aug;18(4):447-476. doi: 10.1007/s10522-017-9685-9. Epub 2017 Mar 3. Biogerontology. 2017. PMID: 28258519 Free PMC article. Review.
-
The role of sirtuins in cardiac disease.Am J Physiol Heart Circ Physiol. 2015 Nov;309(9):H1375-89. doi: 10.1152/ajpheart.00053.2015. Epub 2015 Jul 31. Am J Physiol Heart Circ Physiol. 2015. PMID: 26232232 Free PMC article. Review.
-
Sirtuins: guardians of mammalian healthspan.Trends Genet. 2014 Jul;30(7):271-86. doi: 10.1016/j.tig.2014.04.007. Epub 2014 May 28. Trends Genet. 2014. PMID: 24877878 Free PMC article. Review.
-
Sirtuins and renal diseases: relationship with aging and diabetic nephropathy.Clin Sci (Lond). 2013 Feb;124(3):153-64. doi: 10.1042/CS20120190. Clin Sci (Lond). 2013. PMID: 23075334 Free PMC article. Review.
-
Acetate metabolism and aging: An emerging connection.Mech Ageing Dev. 2010 Jul-Aug;131(7-8):511-6. doi: 10.1016/j.mad.2010.05.001. Epub 2010 May 15. Mech Ageing Dev. 2010. PMID: 20478325
Cited by
-
Are sirtuins viable targets for improving healthspan and lifespan?Nat Rev Drug Discov. 2012 Jun 1;11(6):443-61. doi: 10.1038/nrd3738. Nat Rev Drug Discov. 2012. PMID: 22653216 Free PMC article. Review.
-
A secreted sirtuin from Campylobacter jejuni contributes to neutrophil activation and intestinal inflammation during infection.Sci Adv. 2023 Aug 11;9(32):eade2693. doi: 10.1126/sciadv.ade2693. Epub 2023 Aug 11. Sci Adv. 2023. PMID: 37566649 Free PMC article.
-
Oxidative stress and epigenetic regulation in ageing and age-related diseases.Int J Mol Sci. 2013 Aug 28;14(9):17643-63. doi: 10.3390/ijms140917643. Int J Mol Sci. 2013. PMID: 23989608 Free PMC article. Review.
-
Systemic regulation of mammalian ageing and longevity by brain sirtuins.Nat Commun. 2014 Jun 26;5:4211. doi: 10.1038/ncomms5211. Nat Commun. 2014. PMID: 24967620 Free PMC article. Review.
-
SIRT1 Activation Enhancing 8,3'-Neolignans from the Twigs of Corylopsis coreana Uyeki.Plants (Basel). 2021 Aug 16;10(8):1684. doi: 10.3390/plants10081684. Plants (Basel). 2021. PMID: 34451729 Free PMC article.
References
-
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. - PubMed
-
- Amat R, Planavila A, Chen SL, Iglesias R, Giralt M, Villarroya F. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J Biol Chem. 2009;284:21872–21880. - PMC - PubMed
-
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

