Coupling viruses to dynein and kinesin-1
- PMID: 21878994
- PMCID: PMC3181490
- DOI: 10.1038/emboj.2011.283
Coupling viruses to dynein and kinesin-1
Abstract
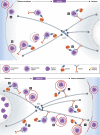
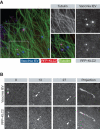
It is now clear that transport on microtubules by dynein and kinesin family motors has an important if not critical role in the replication and spread of many different viruses. Understanding how viruses hijack dynein and kinesin motors using a limited repertoire of proteins offers a great opportunity to determine the molecular basis of motor recruitment. In this review, we discuss the interactions of dynein and kinesin-1 with adenovirus, the α herpes viruses: herpes simplex virus (HSV1) and pseudorabies virus (PrV), human immunodeficiency virus type 1 (HIV-1) and vaccinia virus. We highlight where the molecular links to these opposite polarity motors have been defined and discuss the difficulties associated with identifying viral binding partners where the basis of motor recruitment remains to be established. Ultimately, studying microtubule-based motility of viruses promises to answer fundamental questions as to how the activity and recruitment of the dynein and kinesin-1 motors are coordinated and regulated during bi-directional transport.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Dynamic Dissection of Dynein and Kinesin-1 Cooperatively Mediated Intercellular Transport of Porcine Epidemic Diarrhea Coronavirus along Microtubule Using Single Virus Tracking.Virulence. 2021 Dec;12(1):615-629. doi: 10.1080/21505594.2021.1878748. Virulence. 2021. PMID: 33538234 Free PMC article.
-
Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures.PLoS Pathog. 2010 Jul 8;6(7):e1000991. doi: 10.1371/journal.ppat.1000991. PLoS Pathog. 2010. PMID: 20628567 Free PMC article.
-
Differential regulation of dynein and kinesin motor proteins by tau.Science. 2008 Feb 22;319(5866):1086-9. doi: 10.1126/science.1152993. Epub 2008 Jan 17. Science. 2008. PMID: 18202255 Free PMC article.
-
Kinesin and dynein superfamily proteins and the mechanism of organelle transport.Science. 1998 Jan 23;279(5350):519-26. doi: 10.1126/science.279.5350.519. Science. 1998. PMID: 9438838 Review.
-
Biological Nanomotors with a Revolution, Linear, or Rotation Motion Mechanism.Microbiol Mol Biol Rev. 2016 Jan 27;80(1):161-86. doi: 10.1128/MMBR.00056-15. Print 2016 Mar. Microbiol Mol Biol Rev. 2016. PMID: 26819321 Free PMC article. Review.
Cited by
-
HIV-1 capsids bind and exploit the kinesin-1 adaptor FEZ1 for inward movement to the nucleus.Nat Commun. 2015 Mar 30;6:6660. doi: 10.1038/ncomms7660. Nat Commun. 2015. PMID: 25818806 Free PMC article.
-
Dynein Light-Chain Dynlrb2 Is Essential for Murine Leukemia Virus Traffic and Nuclear Entry.J Virol. 2021 Jul 12;95(15):e0017021. doi: 10.1128/JVI.00170-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 33980598 Free PMC article.
-
West Nile Virus Spreads Transsynaptically within the Pathways of Motor Control: Anatomical and Ultrastructural Mapping of Neuronal Virus Infection in the Primate Central Nervous System.PLoS Negl Trop Dis. 2016 Sep 12;10(9):e0004980. doi: 10.1371/journal.pntd.0004980. eCollection 2016 Sep. PLoS Negl Trop Dis. 2016. PMID: 27617450 Free PMC article.
-
Kinesin-1 Regulates Endocytic Trafficking of Classical Swine Fever Virus along Acetylated Microtubules.J Virol. 2023 Jan 31;97(1):e0192922. doi: 10.1128/jvi.01929-22. Epub 2023 Jan 5. J Virol. 2023. PMID: 36602362 Free PMC article.
-
Microtubule Retrograde Motors and Their Role in Retroviral Transport.Viruses. 2020 Apr 24;12(4):483. doi: 10.3390/v12040483. Viruses. 2020. PMID: 32344581 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

