Valproic acid attenuates proteinuria and kidney injury
- PMID: 21868496
- PMCID: PMC3279948
- DOI: 10.1681/ASN.2010111196
Valproic acid attenuates proteinuria and kidney injury
Abstract
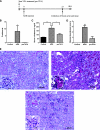
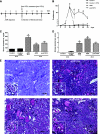
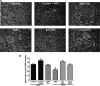
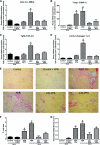
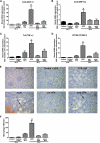
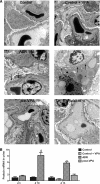
Inhibitors of histone deacetylase (HDAC) have anti-inflammatory and antifibrotic effects in several organs and tissues, but their effect on the progression of renal disease is unknown. Here, we studied the effect of valproic acid in adriamycin-induced nephropathy in mice. Administration of valproic acid before kidney injury prevented the development of proteinuria and the onset of glomerulosclerosis. Even after postponing treatment until the peak of adriamycin-induced proteinuria, valproic acid rapidly decreased the quantity of proteinuria and attenuated the progression of renal disease. Valproic acid abrogated the decrease in glomerular acetylation observed during adriamycin-induced nephropathy. Furthermore, valproic acid attenuated the significant upregulation of profibrotic and proinflammatory genes, the deposition of collagen, and the infiltration of macrophages into the kidney. Valproic acid decreased glomerular apoptosis and proliferation induced by adriamycin. Ultrastructural studies further supported the protective effect of valproic acid on podocytes in this model. Taken together, these data suggest that HDACs contribute to the pathogenesis of renal disease and that HDAC inhibitors may have therapeutic potential in CKD.
Figures

Similar articles
-
Comparison of trichostatin A and valproic acid treatment regimens in a mouse model of kidney fibrosis.Toxicol Appl Pharmacol. 2013 Sep 1;271(2):276-84. doi: 10.1016/j.taap.2013.05.013. Epub 2013 May 22. Toxicol Appl Pharmacol. 2013. PMID: 23707763
-
Rapamycin attenuates the severity of murine adriamycin nephropathy.Am J Nephrol. 2009;29(4):342-52. doi: 10.1159/000166599. Epub 2008 Aug 27. Am J Nephrol. 2009. PMID: 18948688
-
IL-25 induces M2 macrophages and reduces renal injury in proteinuric kidney disease.J Am Soc Nephrol. 2011 Jul;22(7):1229-39. doi: 10.1681/ASN.2010070693. Epub 2011 Jun 30. J Am Soc Nephrol. 2011. PMID: 21719780 Free PMC article.
-
Anti-fibrotic effects of valproic acid: role of HDAC inhibition and associated mechanisms.Epigenomics. 2016 Aug;8(8):1087-101. doi: 10.2217/epi-2016-0034. Epub 2016 Jul 14. Epigenomics. 2016. PMID: 27411759 Review.
-
Adriamycin nephropathy: a model of focal segmental glomerulosclerosis.Nephrology (Carlton). 2011 Jan;16(1):30-8. doi: 10.1111/j.1440-1797.2010.01383.x. Nephrology (Carlton). 2011. PMID: 21175974 Review.
Cited by
-
Chromatin-modifying agents reactivate embryonic renal stem/progenitor genes in human adult kidney epithelial cells but abrogate dedifferentiation and stemness.Cell Reprogram. 2013 Aug;15(4):281-92. doi: 10.1089/cell.2012.0087. Epub 2013 Jul 10. Cell Reprogram. 2013. PMID: 23841748 Free PMC article.
-
Glycosphingolipid GM3 prevents albuminuria and podocytopathy induced by anti-nephrin antibody.Sci Rep. 2022 Sep 26;12(1):16058. doi: 10.1038/s41598-022-20265-w. Sci Rep. 2022. PMID: 36163359 Free PMC article.
-
Valproic acid treatment attenuates cisplatin-induced kidney injury by suppressing proximal tubular cell damage.Clin Transl Sci. 2023 Nov;16(11):2369-2381. doi: 10.1111/cts.13638. Epub 2023 Sep 25. Clin Transl Sci. 2023. PMID: 37700528 Free PMC article.
-
Dynamic changes in histone deacetylases following kidney ischemia-reperfusion injury are critical for promoting proximal tubule proliferation.Am J Physiol Renal Physiol. 2019 May 1;316(5):F875-F888. doi: 10.1152/ajprenal.00499.2018. Epub 2019 Feb 27. Am J Physiol Renal Physiol. 2019. PMID: 30810062 Free PMC article.
-
MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction.J Am Soc Nephrol. 2014 Aug;25(8):1698-709. doi: 10.1681/ASN.2013050527. Epub 2014 Feb 27. J Am Soc Nephrol. 2014. PMID: 24578127 Free PMC article.
References
-
- Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 - PubMed
-
- Chen A, Sheu LF, Ho YS, Lin YF, Chou WY, Chou TC, Lee WH: Experimental focal segmental glomerulosclerosis in mice. Nephron 78: 440–452, 1998 - PubMed
-
- Okuda S, Oh Y, Tsuruda H, Onoyama K, Fujimi S, Fujishima M: Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int 29: 502–510, 1986 - PubMed
-
- Wang Y, Wang YP, Tay YC, Harris DC: Progressive adriamycin nephropathy in mice: Sequence of histologic and immunohistochemical events. Kidney Int 58: 1797–1804, 2000 - PubMed
-
- Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

