Novel proresolving aspirin-triggered DHA pathway
- PMID: 21867913
- PMCID: PMC3164791
- DOI: 10.1016/j.chembiol.2011.06.008
Novel proresolving aspirin-triggered DHA pathway
Abstract
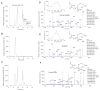
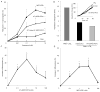
Endogenous mechanisms in the resolution of acute inflammation are of interest because excessive inflammation underlies many pathologic abnormalities. We report an aspirin-triggered DHA metabolome that biosynthesizes a potent product in inflammatory exudates and human leukocytes, namely aspirin-triggered Neuroprotectin D1/Protectin D1 [AT-(NPD1/PD1)]. The complete stereochemistry of AT-(NPD1/PD1) proved to be 10R,17R-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid. The chirality of hydroxyl groups and geometry of the conjugated triene system essential for bioactivity were established by matching biological materials with stereochemically pure isomers prepared by organic synthesis. AT-(NPD1/PD1) reduced neutrophil (PMN) recruitment in murine peritonitis in a dose-dependent fashion whereby neither a Δ(15)-trans-isomer nor DHA was effective. With human cells, AT-(NPD1/PD1) decreased transendothelial PMN migration as well as enhanced efferocytosis of apoptotic human PMN by macrophages. These results indicate that AT-(NPD1/PD1) is a potent anti-inflammatory proresolving molecule.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
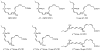


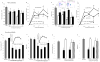
Comment in
-
Aspirin-triggered metabolites of EFAs.Chem Biol. 2011 Oct 28;18(10):1208-9. doi: 10.1016/j.chembiol.2011.10.005. Chem Biol. 2011. PMID: 22035788 Free PMC article.
Similar articles
-
Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes.J Immunol. 2006 Feb 1;176(3):1848-59. doi: 10.4049/jimmunol.176.3.1848. J Immunol. 2006. PMID: 16424216
-
Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions.J Exp Med. 2009 Jan 16;206(1):15-23. doi: 10.1084/jem.20081880. Epub 2008 Dec 22. J Exp Med. 2009. PMID: 19103881 Free PMC article.
-
A newly synthesized 17-epi-NeuroProtectin D1/17-epi-Protectin D1: Authentication and functional regulation of Inflammation-Resolution.Biochem Pharmacol. 2022 Sep;203:115181. doi: 10.1016/j.bcp.2022.115181. Epub 2022 Jul 16. Biochem Pharmacol. 2022. PMID: 35850309 Free PMC article.
-
Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome.Biochim Biophys Acta. 2015 Apr;1851(4):397-413. doi: 10.1016/j.bbalip.2014.08.006. Epub 2014 Aug 17. Biochim Biophys Acta. 2015. PMID: 25139562 Free PMC article. Review.
-
Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection.Prostaglandins Leukot Essent Fatty Acids. 2009 Aug-Sep;81(2-3):205-11. doi: 10.1016/j.plefa.2009.05.024. Epub 2009 Jun 11. Prostaglandins Leukot Essent Fatty Acids. 2009. PMID: 19520558 Free PMC article. Review.
Cited by
-
Stereocontrolled total synthesis of the potent anti-inflammatory and pro-resolving lipid mediator resolvin D3 and its aspirin-triggered 17R-epimer.Org Lett. 2013 Apr 5;15(7):1424-7. doi: 10.1021/ol400484u. Epub 2013 Mar 19. Org Lett. 2013. PMID: 23510485 Free PMC article.
-
Mouse strains and sexual divergence in corneal innervation and nerve regeneration.FASEB J. 2019 Mar;33(3):4598-4609. doi: 10.1096/fj.201801957R. Epub 2018 Dec 18. FASEB J. 2019. PMID: 30561223 Free PMC article.
-
Pro-Resolving Ligands Orchestrate Phagocytosis.Front Immunol. 2021 Jun 10;12:660865. doi: 10.3389/fimmu.2021.660865. eCollection 2021. Front Immunol. 2021. PMID: 34177900 Free PMC article. Review.
-
2016 Russell Ross Memorial Lecture in Vascular Biology: Molecular-Cellular Mechanisms in the Progression of Atherosclerosis.Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):183-189. doi: 10.1161/ATVBAHA.116.308036. Epub 2016 Dec 15. Arterioscler Thromb Vasc Biol. 2017. PMID: 27979856 Free PMC article. Review.
-
Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions.Prog Lipid Res. 2022 Apr;86:101165. doi: 10.1016/j.plipres.2022.101165. Epub 2022 May 1. Prog Lipid Res. 2022. PMID: 35508275 Free PMC article. Review.
References
-
- Antoni C, Braun J. Side effects of anti-TNF therapy: current knowledge. Clin Exp Rheumatol. 2002;20:S152–157. - PubMed
-
- Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. - PubMed
-
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. - PubMed
-
- Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

