Ebola virus entry requires the cholesterol transporter Niemann-Pick C1
- PMID: 21866103
- PMCID: PMC3175325
- DOI: 10.1038/nature10348
Ebola virus entry requires the cholesterol transporter Niemann-Pick C1
Abstract
Infections by the Ebola and Marburg filoviruses cause a rapidly fatal haemorrhagic fever in humans for which no approved antivirals are available. Filovirus entry is mediated by the viral spike glycoprotein (GP), which attaches viral particles to the cell surface, delivers them to endosomes and catalyses fusion between viral and endosomal membranes. Additional host factors in the endosomal compartment are probably required for viral membrane fusion; however, despite considerable efforts, these critical host factors have defied molecular identification. Here we describe a genome-wide haploid genetic screen in human cells to identify host factors required for Ebola virus entry. Our screen uncovered 67 mutations disrupting all six members of the homotypic fusion and vacuole protein-sorting (HOPS) multisubunit tethering complex, which is involved in the fusion of endosomes to lysosomes, and 39 independent mutations that disrupt the endo/lysosomal cholesterol transporter protein Niemann-Pick C1 (NPC1). Cells defective for the HOPS complex or NPC1 function, including primary fibroblasts derived from human Niemann-Pick type C1 disease patients, are resistant to infection by Ebola virus and Marburg virus, but remain fully susceptible to a suite of unrelated viruses. We show that membrane fusion mediated by filovirus glycoproteins and viral escape from the vesicular compartment require the NPC1 protein, independent of its known function in cholesterol transport. Our findings uncover unique features of the entry pathway used by filoviruses and indicate potential antiviral strategies to combat these deadly agents.
Conflict of interest statement
J.E.C., M.R., S.P.W., K.C. and T.R.B. have filed a patent on filovirus host factors identified in this study and T.R.B. is a co-founder of Haplogen, an early-stage company involved in haploid genetic approaches.
Figures
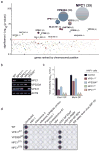
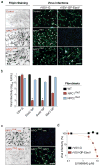
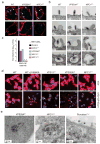
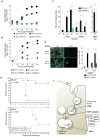
Comment in
-
Achilles heel of Ebola viral entry.Nat Rev Drug Discov. 2011 Sep 30;10(10):731. doi: 10.1038/nrd3568. Nat Rev Drug Discov. 2011. PMID: 21959282 No abstract available.
Similar articles
-
Filoviruses Use the HOPS Complex and UVRAG To Traffic to Niemann-Pick C1 Compartments during Viral Entry.J Virol. 2020 Jul 30;94(16):e01002-20. doi: 10.1128/JVI.01002-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32493822 Free PMC article.
-
Direct Intracellular Visualization of Ebola Virus-Receptor Interaction by In Situ Proximity Ligation.mBio. 2021 Jan 12;12(1):e03100-20. doi: 10.1128/mBio.03100-20. mBio. 2021. PMID: 33436438 Free PMC article.
-
Ebola virus entry requires the host-programmed recognition of an intracellular receptor.EMBO J. 2012 Apr 18;31(8):1947-60. doi: 10.1038/emboj.2012.53. Epub 2012 Mar 6. EMBO J. 2012. PMID: 22395071 Free PMC article.
-
Filovirus entry: a novelty in the viral fusion world.Viruses. 2012 Feb;4(2):258-75. doi: 10.3390/v4020258. Epub 2012 Feb 7. Viruses. 2012. PMID: 22470835 Free PMC article. Review.
-
Potential pharmacological strategies targeting the Niemann-Pick C1 receptor and Ebola virus glycoprotein interaction.Eur J Med Chem. 2021 Nov 5;223:113654. doi: 10.1016/j.ejmech.2021.113654. Epub 2021 Jun 19. Eur J Med Chem. 2021. PMID: 34175537 Review.
Cited by
-
Single-Molecular Förster Resonance Energy Transfer Measurement on Structures and Interactions of Biomolecules.Micromachines (Basel). 2021 Apr 27;12(5):492. doi: 10.3390/mi12050492. Micromachines (Basel). 2021. PMID: 33925350 Free PMC article. Review.
-
Dual microRNA Screens Reveal That the Immune-Responsive miR-181 Promotes Henipavirus Entry and Cell-Cell Fusion.PLoS Pathog. 2016 Oct 26;12(10):e1005974. doi: 10.1371/journal.ppat.1005974. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27783670 Free PMC article.
-
More evidence for widespread antagonistic pleiotropy in polymorphic disease alleles.Front Genet. 2024 Jun 17;15:1404516. doi: 10.3389/fgene.2024.1404516. eCollection 2024. Front Genet. 2024. PMID: 38952711 Free PMC article. Review.
-
Addressing Therapeutic Options for Ebola Virus Infection in Current and Future Outbreaks.Antimicrob Agents Chemother. 2015 Oct;59(10):5892-902. doi: 10.1128/AAC.01105-15. Epub 2015 Jul 27. Antimicrob Agents Chemother. 2015. PMID: 26248374 Free PMC article. Review.
-
Neutralizing Antibody and Soluble ACE2 Inhibition of a Replication-Competent VSV-SARS-CoV-2 and a Clinical Isolate of SARS-CoV-2.Cell Host Microbe. 2020 Sep 9;28(3):475-485.e5. doi: 10.1016/j.chom.2020.06.021. Epub 2020 Jul 3. Cell Host Microbe. 2020. PMID: 32735849 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- AI081842/AI/NIAID NIH HHS/United States
- U54 AI057159/AI/NIAID NIH HHS/United States
- R21 HG004938/HG/NHGRI NIH HHS/United States
- R01 AI088027-03/AI/NIAID NIH HHS/United States
- R01 AI088027/AI/NIAID NIH HHS/United States
- R56 AI081842/AI/NIAID NIH HHS/United States
- T32 AI070117/AI/NIAID NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- R21 HG004938-01/HG/NHGRI NIH HHS/United States
- R01 AI081842/AI/NIAID NIH HHS/United States
- R01 AI081842-03/AI/NIAID NIH HHS/United States
- U54 AI057159-09/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

