Natural killer cell activation by dendritic cells: balancing inhibitory and activating signals
- PMID: 21861182
- PMCID: PMC11114903
- DOI: 10.1007/s00018-011-0801-8
Natural killer cell activation by dendritic cells: balancing inhibitory and activating signals
Abstract
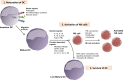
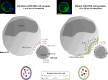
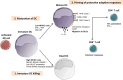
Natural killer (NK) cells have originally been identified by their spontaneous cytolytic potential against tumor cells, which, however, might result from pre-activation due to prior pathogen exposure. Resting NK cells, on the contrary, require activation by bystander antigen-presenting cells to reach their full functional competence. In this review, we will summarize studies on how dendritic cells (DCs), the most potent type of antigen-presenting cell, communicate with human NK cells to activate them in secondary lymphoid organs and to integrate signals from activated NK cells at sites of inflammation for their own maturation. Furthermore, we will review aspects of the immunological synapse, which mediates this cross-talk. These studies provide the mechanistic understanding of how mature DCs can activate NK cells and survive to go on for the activation of adaptive immunity. This feature of DCs, to activate different waves of immune responses, could be harnessed for immunotherapies, including vaccinations.
Figures
Similar articles
-
Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells.J Exp Med. 2002 Feb 4;195(3):343-51. doi: 10.1084/jem.20011149. J Exp Med. 2002. PMID: 11828009 Free PMC article.
-
Natural killer-dendritic cell cross-talk in cancer immunotherapy.Expert Opin Biol Ther. 2005 Oct;5(10):1303-15. doi: 10.1517/14712598.5.10.1303. Expert Opin Biol Ther. 2005. PMID: 16197336 Review.
-
Cytoskeletal stabilization of inhibitory interactions in immunologic synapses of mature human dendritic cells with natural killer cells.Blood. 2011 Dec 15;118(25):6487-98. doi: 10.1182/blood-2011-07-366328. Epub 2011 Sep 13. Blood. 2011. PMID: 21917751 Free PMC article.
-
Dendritic cell interactions with NK cells from different tissues.J Clin Immunol. 2009 May;29(3):265-73. doi: 10.1007/s10875-009-9283-y. Epub 2009 Mar 12. J Clin Immunol. 2009. PMID: 19280325 Review.
-
Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection.J Immunol. 2004 Nov 15;173(10):6072-81. doi: 10.4049/jimmunol.173.10.6072. J Immunol. 2004. PMID: 15528343
Cited by
-
Trans-endocytosis of intact IL-15Rα-IL-15 complex from presenting cells into NK cells favors signaling for proliferation.Proc Natl Acad Sci U S A. 2020 Jan 7;117(1):522-531. doi: 10.1073/pnas.1911678117. Epub 2019 Dec 23. Proc Natl Acad Sci U S A. 2020. PMID: 31871169 Free PMC article.
-
NK cells are necessary for recovery of corneal CD11c+ dendritic cells after epithelial abrasion injury.J Leukoc Biol. 2013 Aug;94(2):343-51. doi: 10.1189/jlb.1212633. Epub 2013 May 21. J Leukoc Biol. 2013. PMID: 23695308 Free PMC article.
-
Induction of potent NK cell-dependent anti-myeloma cytotoxic T cells in response to combined mapatumumab and bortezomib.Oncoimmunology. 2015 May 27;4(9):e1038011. doi: 10.1080/2162402X.2015.1038011. eCollection 2015 Sep. Oncoimmunology. 2015. PMID: 26405606 Free PMC article.
-
Clinically applicable CD34+-derived blood dendritic cell subsets exhibit key subset-specific features and potently boost anti-tumor T and NK cell responses.Cancer Immunol Immunother. 2021 Nov;70(11):3167-3181. doi: 10.1007/s00262-021-02899-3. Epub 2021 Apr 1. Cancer Immunol Immunother. 2021. PMID: 33796917 Free PMC article.
-
Modeling putative therapeutic implications of exosome exchange between tumor and immune cells.Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):E4165-74. doi: 10.1073/pnas.1416745111. Epub 2014 Sep 22. Proc Natl Acad Sci U S A. 2014. PMID: 25246552 Free PMC article.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

