Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway
- PMID: 21858054
- PMCID: PMC3156119
- DOI: 10.1371/journal.pone.0023278
Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway
Abstract
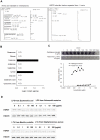
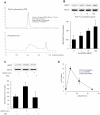
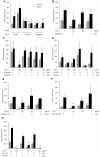
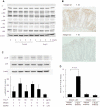
Probiotics exhibit beneficial effects on human health, particularly in the maintenance of intestinal homeostasis in a complex manner notwithstanding the diversity of an intestinal flora between individuals. Thus, it is highly probable that some common molecules secreted by probiotic and/or commensal bacteria contribute to the maintenance of intestinal homeostasis and protect the intestinal epithelium from injurious stimuli. To address this question, we aimed to isolate the cytoprotective compound from a lactobacillus strain, Lactobacillus brevis SBC8803 which possess the ability to induce cytoprotective heat shock proteins in mouse small intestine. L. brevis was incubated in MRS broth and the supernatant was passed through with a 0.2-µm filter. Caco2/bbe cells were treated with the culture supernatant, and HSP27 expression was evaluated by Western blotting. HSP27-inducible components were separated by ammonium sulfate precipitation, DEAE anion exchange chromatography, gel filtration, and HPLC. Finally, we identified that the HSP27-inducible fraction was polyphosphate (poly P), a simple repeated structure of phosphates, which is a common product of lactobacilli and other bacteria associated with intestinal microflora without any definitive physiological functions. Then, poly P was synthesized by poly P-synthesizing enzyme polyphosphate kinase. The synthesized poly P significantly induced HSP27 from Caco2/BBE cells. In addition, Poly P suppressed the oxidant-induced intestinal permeability in the mouse small intestine and pharmacological inhibitors of p38 MAPK and integrins counteract its protective effect. Daily intrarectal administration of poly P (10 µg) improved the inflammation grade and survival rate in 4% sodium dextran sulfate-administered mice. This study, for the first time, demonstrated that poly P is the molecule responsible for maintaining intestinal barrier actions which are mediated through the intestinal integrin β1-p38 MAPK.
Conflict of interest statement
Figures




Similar articles
-
Heat-killed body of lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function.Inflamm Bowel Dis. 2011 Nov;17(11):2235-50. doi: 10.1002/ibd.21597. Epub 2011 Jan 6. Inflamm Bowel Dis. 2011. PMID: 21987297
-
Polyphosphate, an active molecule derived from probiotic Lactobacillus brevis, improves the fibrosis in murine colitis.Transl Res. 2015 Aug;166(2):163-75. doi: 10.1016/j.trsl.2015.02.002. Epub 2015 Feb 19. Transl Res. 2015. PMID: 25766132
-
Probiotic-derived polyphosphate improves the intestinal barrier function through the caveolin-dependent endocytic pathway.Biochem Biophys Res Commun. 2015 Nov 20;467(3):541-8. doi: 10.1016/j.bbrc.2015.09.159. Epub 2015 Oct 13. Biochem Biophys Res Commun. 2015. PMID: 26459590
-
AMP-18 facilitates assembly and stabilization of tight junctions to protect the colonic mucosal barrier.Inflamm Bowel Dis. 2012 Sep;18(9):1749-59. doi: 10.1002/ibd.22886. Epub 2012 Jan 23. Inflamm Bowel Dis. 2012. PMID: 22271547 Free PMC article.
-
Flavaglines Ameliorate Experimental Colitis and Protect Against Intestinal Epithelial Cell Apoptosis and Mitochondrial Dysfunction.Inflamm Bowel Dis. 2016 Jan;22(1):55-67. doi: 10.1097/MIB.0000000000000592. Inflamm Bowel Dis. 2016. PMID: 26398710 Free PMC article.
Cited by
-
Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology.World J Gastrointest Pathophysiol. 2012 Feb 15;3(1):27-43. doi: 10.4291/wjgp.v3.i1.27. World J Gastrointest Pathophysiol. 2012. PMID: 22368784 Free PMC article.
-
Inorganic Polyphosphates As Storage for and Generator of Metabolic Energy in the Extracellular Matrix.Chem Rev. 2019 Dec 26;119(24):12337-12374. doi: 10.1021/acs.chemrev.9b00460. Epub 2019 Nov 18. Chem Rev. 2019. PMID: 31738523 Free PMC article. Review.
-
Polyphosphate as a Target for Interference With Inflammation and Thrombosis.Front Med (Lausanne). 2019 Apr 12;6:76. doi: 10.3389/fmed.2019.00076. eCollection 2019. Front Med (Lausanne). 2019. PMID: 31106204 Free PMC article. Review.
-
Antioxidant Activity of Yogurt Fermented at Low Temperature and Its Anti-inflammatory Effect on DSS-induced Colitis in Mice.Food Sci Anim Resour. 2019 Feb;39(1):162-176. doi: 10.5851/kosfa.2019.e13. Epub 2019 Feb 28. Food Sci Anim Resour. 2019. PMID: 30882084 Free PMC article.
-
Early changes in microbial colonization selectively modulate intestinal enzymes, but not inducible heat shock proteins in young adult Swine.PLoS One. 2014 Feb 4;9(2):e87967. doi: 10.1371/journal.pone.0087967. eCollection 2014. PLoS One. 2014. PMID: 24505340 Free PMC article.
References
-
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. - PubMed
-
- De Keersmaecker SC, Verhoeven TL, Desair J, Marchal K, Vanderleyden J, et al. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett. 2006;259:89–96. - PubMed
-
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, et al. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

