IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis
- PMID: 21858022
- PMCID: PMC3156735
- DOI: 10.1371/journal.pone.0023185
IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis
Abstract
Background: Idiopathic pulmonary fibrosis is a devastating as yet untreatable disease. We demonstrated recently the predominant role of the NLRP3 inflammasome activation and IL-1β expression in the establishment of pulmonary inflammation and fibrosis in mice.
Methods: The contribution of IL-23 or IL-17 in pulmonary inflammation and fibrosis was assessed using the bleomycin model in deficient mice.
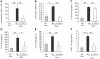
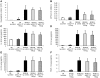
Results: We show that bleomycin or IL-1β-induced lung injury leads to increased expression of early IL-23p19, and IL-17A or IL-17F expression. Early IL-23p19 and IL-17A, but not IL-17F, and IL-17RA signaling are required for inflammatory response to BLM as shown with gene deficient mice or mice treated with neutralizing antibodies. Using FACS analysis, we show a very early IL-17A and IL-17F expression by RORγt(+) γδ T cells and to a lesser extent by CD4αβ(+) T cells, but not by iNKT cells, 24 hrs after BLM administration. Moreover, IL-23p19 and IL-17A expressions or IL-17RA signaling are necessary to pulmonary TGF-β1 production, collagen deposition and evolution to fibrosis.
Conclusions: Our findings demonstrate the existence of an early IL-1β-IL-23-IL-17A axis leading to pulmonary inflammation and fibrosis and identify innate IL-23 and IL-17A as interesting drug targets for IL-1β driven lung pathology.
Conflict of interest statement
Figures

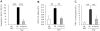


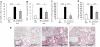

Similar articles
-
Th17 cells and IL-17 promote the skin and lung inflammation and fibrosis process in a bleomycin-induced murine model of systemic sclerosis.Clin Exp Rheumatol. 2016 Sep-Oct;34 Suppl 100(5):14-22. Epub 2016 Jan 11. Clin Exp Rheumatol. 2016. PMID: 26750756
-
Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms.J Immunol. 2011 Sep 15;187(6):3003-14. doi: 10.4049/jimmunol.1004081. Epub 2011 Aug 12. J Immunol. 2011. PMID: 21841134
-
Elevated frequencies of CD4(+) IL-21(+) T, CD4(+) IL-21R(+) T and IL-21(+) Th17 cells, and increased levels of IL-21 in bleomycin-induced mice may be associated with dermal and pulmonary inflammation and fibrosis.Int J Rheum Dis. 2016 Apr;19(4):392-404. doi: 10.1111/1756-185X.12522. Epub 2014 Dec 25. Int J Rheum Dis. 2016. PMID: 25545680
-
Pulmonary fibrosis and type-17 immunity.Respir Investig. 2023 Sep;61(5):553-562. doi: 10.1016/j.resinv.2023.05.005. Epub 2023 Jun 23. Respir Investig. 2023. PMID: 37356133 Review.
-
IL-17 and IL-17-producing cells in protection versus pathology.Nat Rev Immunol. 2023 Jan;23(1):38-54. doi: 10.1038/s41577-022-00746-9. Epub 2022 Jul 5. Nat Rev Immunol. 2023. PMID: 35790881 Free PMC article. Review.
Cited by
-
Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation.J Leukoc Biol. 2016 Aug;100(2):381-9. doi: 10.1189/jlb.3A1015-474R. Epub 2016 Apr 5. J Leukoc Biol. 2016. PMID: 27049023 Free PMC article.
-
The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis.Int J Mol Sci. 2019 Aug 8;20(16):3876. doi: 10.3390/ijms20163876. Int J Mol Sci. 2019. PMID: 31398940 Free PMC article. Review.
-
IL-17A mediates early post-transplant lesions after heterotopic trachea allotransplantation in Mice.PLoS One. 2013 Jul 30;8(7):e70236. doi: 10.1371/journal.pone.0070236. Print 2013. PLoS One. 2013. PMID: 23936171 Free PMC article.
-
IL-17 in the lung: the good, the bad, and the ugly.Am J Physiol Lung Cell Mol Physiol. 2018 Jan 1;314(1):L6-L16. doi: 10.1152/ajplung.00344.2017. Epub 2017 Aug 31. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 28860146 Free PMC article. Review.
-
Regulation of inflammatory biomarkers by intravenous methylprednisolone in pediatric ARDS patients: Results from a double-blind, placebo-controlled randomized pilot trial.Cytokine. 2016 Jan;77:63-71. doi: 10.1016/j.cyto.2015.10.007. Epub 2015 Nov 3. Cytokine. 2016. PMID: 26545141 Free PMC article. Clinical Trial.
References
-
- Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103:1808–1812. - PubMed
-
- Gasse P, Riteau N, Charron S, Girre S, Fick L, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. - PubMed
-
- Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. - PubMed
-
- Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, et al. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–4672. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

