ATR-p53 restricts homologous recombination in response to replicative stress but does not limit DNA interstrand crosslink repair in lung cancer cells
- PMID: 21857991
- PMCID: PMC3155521
- DOI: 10.1371/journal.pone.0023053
ATR-p53 restricts homologous recombination in response to replicative stress but does not limit DNA interstrand crosslink repair in lung cancer cells
Abstract
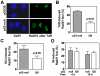
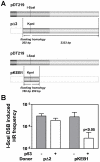
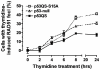
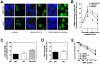
Homologous recombination (HR) is required for the restart of collapsed DNA replication forks and error-free repair of DNA double-strand breaks (DSB). However, unscheduled or hyperactive HR may lead to genomic instability and promote cancer development. The cellular factors that restrict HR processes in mammalian cells are only beginning to be elucidated. The tumor suppressor p53 has been implicated in the suppression of HR though it has remained unclear why p53, as the guardian of the genome, would impair an error-free repair process. Here, we show for the first time that p53 downregulates foci formation of the RAD51 recombinase in response to replicative stress in H1299 lung cancer cells in a manner that is independent of its role as a transcription factor. We find that this downregulation of HR is not only completely dependent on the binding site of p53 with replication protein A but also the ATR/ATM serine 15 phosphorylation site. Genetic analysis suggests that ATR but not ATM kinase modulates p53's function in HR. The suppression of HR by p53 can be bypassed under experimental conditions that cause DSB either directly or indirectly, in line with p53's role as a guardian of the genome. As a result, transactivation-inactive p53 does not compromise the resistance of H1299 cells to the interstrand crosslinking agent mitomycin C. Altogether, our data support a model in which p53 plays an anti-recombinogenic role in the ATR-dependent mammalian replication checkpoint but does not impair a cell's ability to use HR for the removal of DSB induced by cytotoxic agents.
Conflict of interest statement
Figures


Similar articles
-
Decarbamoyl mitomycin C (DMC) activates p53-independent ataxia telangiectasia and rad3 related protein (ATR) chromatin eviction.Cell Cycle. 2015;14(5):744-54. doi: 10.1080/15384101.2014.997517. Cell Cycle. 2015. PMID: 25565400 Free PMC article.
-
Activation of the S phase DNA damage checkpoint by mitomycin C.J Cell Physiol. 2007 May;211(2):468-76. doi: 10.1002/jcp.20957. J Cell Physiol. 2007. PMID: 17167777
-
ATR and H2AX cooperate in maintaining genome stability under replication stress.J Biol Chem. 2009 Feb 27;284(9):5994-6003. doi: 10.1074/jbc.M806739200. Epub 2008 Dec 2. J Biol Chem. 2009. PMID: 19049966 Free PMC article.
-
p53's double life: transactivation-independent repression of homologous recombination.Trends Genet. 2004 Jun;20(6):235-43. doi: 10.1016/j.tig.2004.04.003. Trends Genet. 2004. PMID: 15145576 Review.
-
The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review.
Cited by
-
Transcriptional activation of p21Waf1 contributes to suppression of HR by p53 in response to replication arrest induced by camptothecin.Oncotarget. 2018 May 22;9(39):25427-25440. doi: 10.18632/oncotarget.25172. eCollection 2018 May 22. Oncotarget. 2018. PMID: 29875999 Free PMC article.
-
DNA repair pathway gene expression score correlates with repair proficiency and tumor sensitivity to chemotherapy.Sci Transl Med. 2014 Mar 26;6(229):229ra42. doi: 10.1126/scitranslmed.3008291. Sci Transl Med. 2014. PMID: 24670686 Free PMC article.
-
Ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase inhibition is synthetically lethal in XRCC1 deficient ovarian cancer cells.PLoS One. 2013;8(2):e57098. doi: 10.1371/journal.pone.0057098. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451157 Free PMC article.
-
Integrin α6β4 signals through DNA damage response pathway to sensitize breast cancer cells to cisplatin.Front Oncol. 2022 Nov 10;12:1043538. doi: 10.3389/fonc.2022.1043538. eCollection 2022. Front Oncol. 2022. PMID: 36439467 Free PMC article.
-
Alterations in the p53 isoform ratio govern breast cancer cell fate in response to DNA damage.Cell Death Dis. 2022 Oct 28;13(10):907. doi: 10.1038/s41419-022-05349-9. Cell Death Dis. 2022. PMID: 36307393 Free PMC article.
References
-
- Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6:44–55. - PubMed
-
- Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst) 2007;6:923–935. - PubMed
-
- Reliene R, Bishop AJ, Schiestl RH. Involvement of homologous recombination in carcinogenesis. Adv Genet. 2007;58:67–87. - PubMed
-
- Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

