Opposing activities of LIT-1/NLK and DAF-6/patched-related direct sensory compartment morphogenesis in C. elegans
- PMID: 21857800
- PMCID: PMC3153439
- DOI: 10.1371/journal.pbio.1001121
Opposing activities of LIT-1/NLK and DAF-6/patched-related direct sensory compartment morphogenesis in C. elegans
Abstract
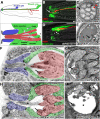
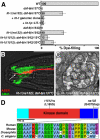
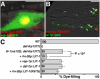
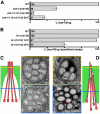
Glial cells surround neuronal endings to create enclosed compartments required for neuronal function. This architecture is seen at excitatory synapses and at sensory neuron receptive endings. Despite the prevalence and importance of these compartments, how they form is not known. We used the main sensory organ of C. elegans, the amphid, to investigate this issue. daf-6/Patched-related is a glia-expressed gene previously implicated in amphid sensory compartment morphogenesis. By comparing time series of electron-microscopy (EM) reconstructions of wild-type and daf-6 mutant embryos, we show that daf-6 acts to restrict compartment size. From a genetic screen, we found that mutations in the gene lit-1/Nemo-like kinase (NLK) suppress daf-6. EM and genetic studies demonstrate that lit-1 acts within glia, in counterbalance to daf-6, to promote sensory compartment expansion. Although LIT-1 has been shown to regulate Wnt signaling, our genetic studies demonstrate a novel, Wnt-independent role for LIT-1 in sensory compartment size control. The LIT-1 activator MOM-4/TAK1 is also important for compartment morphogenesis and both proteins line the glial sensory compartment. LIT-1 compartment localization is important for its function and requires neuronal signals. Furthermore, the conserved LIT-1 C-terminus is necessary and sufficient for this localization. Two-hybrid and co-immunoprecipitation studies demonstrate that the LIT-1 C-terminus binds both actin and the Wiskott-Aldrich syndrome protein (WASP), an actin regulator. We use fluorescence light microscopy and fluorescence EM methodology to show that actin is highly enriched around the amphid sensory compartment. Finally, our genetic studies demonstrate that WASP is important for compartment expansion and functions in the same pathway as LIT-1. The studies presented here uncover a novel, Wnt-independent role for the conserved Nemo-like kinase LIT-1 in controlling cell morphogenesis in conjunction with the actin cytoskeleton. Our results suggest that the opposing daf-6 and lit-1 glial pathways act together to control sensory compartment size.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
IGDB-2, an Ig/FNIII protein, binds the ion channel LGC-34 and controls sensory compartment morphogenesis in C. elegans.Dev Biol. 2017 Oct 1;430(1):105-112. doi: 10.1016/j.ydbio.2017.08.009. Epub 2017 Aug 10. Dev Biol. 2017. PMID: 28803967 Free PMC article.
-
Some, but not all, retromer components promote morphogenesis of C. elegans sensory compartments.Dev Biol. 2012 Feb 1;362(1):42-9. doi: 10.1016/j.ydbio.2011.11.009. Epub 2011 Nov 23. Dev Biol. 2012. PMID: 22138055 Free PMC article.
-
On the morphogenesis of glial compartments in the sensory organs of Caenorhabditis elegans.Worm. 2012 Jan 1;1(1):51-5. doi: 10.4161/worm.19343. Worm. 2012. PMID: 24058823 Free PMC article.
-
Actin-based forces driving embryonic morphogenesis in Caenorhabditis elegans.Curr Opin Genet Dev. 2006 Aug;16(4):392-8. doi: 10.1016/j.gde.2006.06.002. Epub 2006 Jun 16. Curr Opin Genet Dev. 2006. PMID: 16782324 Review.
-
Imaging Epidermal Cell Rearrangement in the C. elegans Embryo.Methods Mol Biol. 2022;2438:345-376. doi: 10.1007/978-1-0716-2035-9_22. Methods Mol Biol. 2022. PMID: 35147953 Free PMC article. Review.
Cited by
-
[The effect of glial cells in the function and development of the nervous system in Caenorhabditis elegans].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016 May 25;45(3):315-22. doi: 10.3785/j.issn.1008-9292.2016.05.16. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 27651199 Free PMC article. Chinese.
-
Caenorhabditis elegans PTR/PTCHD PTR-18 promotes the clearance of extracellular hedgehog-related protein via endocytosis.PLoS Genet. 2021 Apr 19;17(4):e1009457. doi: 10.1371/journal.pgen.1009457. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33872306 Free PMC article.
-
Neuron cilia restrain glial KCC-3 to a microdomain to regulate multisensory processing.Cell Rep. 2024 Mar 26;43(3):113844. doi: 10.1016/j.celrep.2024.113844. Epub 2024 Feb 27. Cell Rep. 2024. PMID: 38421867 Free PMC article.
-
The ClC Cl- channel CLH-1 mediates HCO3 - efflux from the amphid sheath glia in C. elegans.MicroPubl Biol. 2022 Jan 12;2022:10.17912/micropub.biology.000510. doi: 10.17912/micropub.biology.000510. eCollection 2022. MicroPubl Biol. 2022. PMID: 35047763 Free PMC article.
-
The Caenorhabditis elegans Patched domain protein PTR-4 is required for proper organization of the precuticular apical extracellular matrix.Genetics. 2021 Nov 5;219(3):iyab132. doi: 10.1093/genetics/iyab132. Genetics. 2021. PMID: 34740248 Free PMC article.
References
-
- Burkitt G. H, Young B, Heath J. W. New York: Churchill Livinstone; 1993. Wheater's functional histology: a text and colour atlas.
-
- Ross M. H, Romrell L. J, Kaye G. I. Baltimore: Williams & Wilkins; 1995. Histology: a text and atlas.
-
- Bell J, Bolanowski S, Holmes M. H. The structure and function of Pacinian corpuscles: a review. Prog Neurobiol. 1994;42:79–128. - PubMed
-
- Suzuki Y, Takeda M, Farbman A. I. Supporting cells as phagocytes in the olfactory epithelium after bulbectomy. J Comp Neurol. 1996;376:509–517. - PubMed
-
- Hansel D. E, Eipper B. A, Ronnett G. V. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

