Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response
- PMID: 21852455
- PMCID: PMC3202989
- DOI: 10.1126/science.1209126
Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response
Abstract
The unfolded protein response (UPR) detects the accumulation of unfolded proteins in the endoplasmic reticulum (ER) and adjusts the protein-folding capacity to the needs of the cell. Under conditions of ER stress, the transmembrane protein Ire1 oligomerizes to activate its cytoplasmic kinase and ribonuclease domains. It is unclear what feature of ER stress Ire1 detects. We found that the core ER-lumenal domain (cLD) of yeast Ire1 binds to unfolded proteins in yeast cells and to peptides primarily composed of basic and hydrophobic residues in vitro. Mutation of amino acid side chains exposed in a putative peptide-binding groove of Ire1 cLD impaired peptide binding. Peptide binding caused Ire1 cLD oligomerization in vitro, suggesting that direct binding to unfolded proteins activates the UPR.
Figures

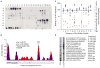
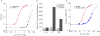
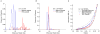
Comment in
-
Cell biology. Sensing ER stress.Science. 2011 Sep 30;333(6051):1830-1. doi: 10.1126/science.1212840. Science. 2011. PMID: 21960615 No abstract available.
Similar articles
-
On the mechanism of sensing unfolded protein in the endoplasmic reticulum.Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):18773-84. doi: 10.1073/pnas.0509487102. Epub 2005 Dec 19. Proc Natl Acad Sci U S A. 2005. PMID: 16365312 Free PMC article.
-
BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response.PLoS Biol. 2010 Jul 6;8(7):e1000415. doi: 10.1371/journal.pbio.1000415. PLoS Biol. 2010. PMID: 20625545 Free PMC article.
-
Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins.J Cell Biol. 2007 Oct 8;179(1):75-86. doi: 10.1083/jcb.200704166. J Cell Biol. 2007. PMID: 17923530 Free PMC article.
-
How transmembrane proteins sense endoplasmic reticulum stress.Antioxid Redox Signal. 2007 Dec;9(12):2295-303. doi: 10.1089/ars.2007.1819. Antioxid Redox Signal. 2007. PMID: 17896870 Review.
-
How IRE1 reacts to ER stress.Cell. 2008 Jan 11;132(1):24-6. doi: 10.1016/j.cell.2007.12.017. Cell. 2008. PMID: 18191217 Review.
Cited by
-
Chromatin proteins: key responders to stress.PLoS Biol. 2012;10(7):e1001371. doi: 10.1371/journal.pbio.1001371. Epub 2012 Jul 31. PLoS Biol. 2012. PMID: 22859908 Free PMC article.
-
IRE1: ER stress sensor and cell fate executor.Trends Cell Biol. 2013 Nov;23(11):547-55. doi: 10.1016/j.tcb.2013.06.005. Epub 2013 Jul 21. Trends Cell Biol. 2013. PMID: 23880584 Free PMC article. Review.
-
Potential for therapeutic manipulation of the UPR in disease.Semin Immunopathol. 2013 May;35(3):351-73. doi: 10.1007/s00281-013-0370-z. Epub 2013 Apr 10. Semin Immunopathol. 2013. PMID: 23572207 Free PMC article. Review.
-
Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in plants.Protoplasma. 2016 May;253(3):753-764. doi: 10.1007/s00709-015-0842-1. Epub 2015 Jun 10. Protoplasma. 2016. PMID: 26060134 Review.
-
Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes.J Mol Biol. 2020 Mar 6;432(5):1514-1534. doi: 10.1016/j.jmb.2019.09.016. Epub 2019 Oct 16. J Mol Biol. 2020. PMID: 31628942 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

