Inhibition of retromer activity by herpesvirus saimiri tip leads to CD4 downregulation and efficient T cell transformation
- PMID: 21849449
- PMCID: PMC3187508
- DOI: 10.1128/JVI.00757-11
Inhibition of retromer activity by herpesvirus saimiri tip leads to CD4 downregulation and efficient T cell transformation
Abstract
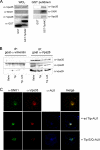
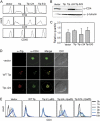
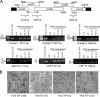
The mammalian retromer is an evolutionally conserved protein complex composed of a vacuolar protein sorting trimer (Vps 26/29/35) that participates in cargo recognition and a sorting nexin (SNX) dimer that binds to endosomal membranes. The retromer plays an important role in efficient retrograde transport for endosome-to-Golgi retrieval of the cation-independent mannose-6-phosphate receptor (CI-MPR), a receptor for lysosomal hydrolases, and other endosomal proteins. This ultimately contributes to the control of cell growth, cell adhesion, and cell migration. The herpesvirus saimiri (HVS) tyrosine kinase-interacting protein (Tip), required for the immortalization of primary T lymphocytes, targets cellular signaling molecules, including Lck tyrosine kinases and the p80 endosomal trafficking protein. Despite the pronounced effects of HVS Tip on T cell signal transduction, the details of its activity on T cell immortalization remain elusive. Here, we report that the amino-terminal conserved, glutamate-rich sequence of Tip specifically interacts with the retromer subunit Vps35 and that this interaction not only causes the redistribution of Vps35 from the early endosome to the lysosome but also drastically inhibits retromer activity, as measured by decreased levels of CI-MPR and lower activities of cellular lysosomal hydrolases. Physiologically, the inhibition of intracellular retromer activity by Tip is ultimately linked to the downregulation of CD4 surface expression and to the efficient in vitro immortalization of primary human T cells to interleukin-2 (IL-2)-independent permanent growth. Therefore, HVS Tip uniquely targets the retromer complex to impair the intracellular trafficking functions of infected cells, ultimately contributing to efficient T cell transformation.
Figures

Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I.Elife. 2020 May 20;9:e55845. doi: 10.7554/eLife.55845. Elife. 2020. PMID: 32432547 Free PMC article.
-
A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion.Nat Cell Biol. 2011 Jul 3;13(8):914-923. doi: 10.1038/ncb2281. Nat Cell Biol. 2011. PMID: 21725319 Free PMC article.
-
Australia in 2030: what is our path to health for all?Med J Aust. 2021 May;214 Suppl 8:S5-S40. doi: 10.5694/mja2.51020. Med J Aust. 2021. PMID: 33934362
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
Cited by
-
Genome-wide siRNA screen identifies the retromer as a cellular entry factor for human papillomavirus.Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7452-7. doi: 10.1073/pnas.1302164110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569269 Free PMC article.
-
The retromer complex - endosomal protein recycling and beyond.J Cell Sci. 2012 Oct 15;125(Pt 20):4693-702. doi: 10.1242/jcs.103440. Epub 2012 Nov 12. J Cell Sci. 2012. PMID: 23148298 Free PMC article. Review.
-
Antigen processing and remodeling of the endosomal pathway: requirements for antigen cross-presentation.Front Immunol. 2012 Mar 7;3:37. doi: 10.3389/fimmu.2012.00037. eCollection 2012. Front Immunol. 2012. PMID: 22566920 Free PMC article.
-
γ-secretase facilitates retromer-mediated retrograde transport.bioRxiv [Preprint]. 2024 Jun 7:2024.06.07.597932. doi: 10.1101/2024.06.07.597932. bioRxiv. 2024. PMID: 38895404 Free PMC article. Preprint.
-
The human PDZome: a gateway to PSD95-Disc large-zonula occludens (PDZ)-mediated functions.Mol Cell Proteomics. 2013 Sep;12(9):2587-603. doi: 10.1074/mcp.O112.021022. Epub 2013 May 30. Mol Cell Proteomics. 2013. PMID: 23722234 Free PMC article.
References
-
- Aiken C., Konner J., Landau N. R., Lenburg M. E., Trono D. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76: 853–864 - PubMed
-
- Biesinger B., et al. 1995. The product of the Herpesvirus saimiri open reading frame 1 (tip) interacts with T cell-specific kinase p56lck in transformed cells. J. Biol. Chem. 270: 4729–4734 - PubMed
-
- Braulke T., Mach L., Hoflack B., Glossl J. 1992. Biosynthesis and endocytosis of lysosomal enzymes in human colon carcinoma SW 1116 cells: impaired internalization of plasma membrane-associated cation-independent mannose 6-phosphate receptor. Arch. Biochem. Biophys. 298: 176–181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

