Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus
- PMID: 21844353
- PMCID: PMC3167523
- DOI: 10.1073/pnas.1110133108
Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus
Abstract
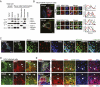
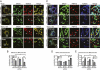
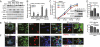
RIG-I is a cytosolic pathogen recognition receptor that engages viral RNA in infected cells to trigger innate immune defenses through its adaptor protein MAVS. MAVS resides on mitochondria and peroxisomes, but how its signaling is coordinated among these organelles has not been defined. Here we show that a major site of MAVS signaling is the mitochondrial-associated membrane (MAM), a distinct membrane compartment that links the endoplasmic reticulum to mitochondria. During RNA virus infection, RIG-I is recruited to the MAM to bind MAVS. Dynamic MAM tethering to mitochondria and peroxisomes then coordinates MAVS localization to form a signaling synapse between membranes. Importantly, the hepatitis C virus NS3/4A protease, which cleaves MAVS to support persistent infection, targets this synapse for MAVS proteolysis from the MAM, but not from mitochondria, to ablate RIG-I signaling of immune defenses. Thus, the MAM mediates an intracellular immune synapse that directs antiviral innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Control of innate immune signaling and membrane targeting by the Hepatitis C virus NS3/4A protease are governed by the NS3 helix α0.J Virol. 2012 Mar;86(6):3112-20. doi: 10.1128/JVI.06727-11. Epub 2012 Jan 11. J Virol. 2012. PMID: 22238314 Free PMC article.
-
Hepatitis C virus NS3-4A inhibits the peroxisomal MAVS-dependent antiviral signalling response.J Cell Mol Med. 2016 Apr;20(4):750-7. doi: 10.1111/jcmm.12801. Epub 2016 Feb 10. J Cell Mol Med. 2016. PMID: 26865163 Free PMC article.
-
Hepacivirus NS3/4A Proteases Interfere with MAVS Signaling in both Their Cognate Animal Hosts and Humans: Implications for Zoonotic Transmission.J Virol. 2016 Nov 14;90(23):10670-10681. doi: 10.1128/JVI.01634-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27654291 Free PMC article.
-
[Chronic hepatitis C virus infection attenuates host antiviral innate immune response].Nihon Rinsho. 2015 Feb;73(2):234-8. Nihon Rinsho. 2015. PMID: 25764676 Review. Japanese.
-
Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter.Curr Opin Immunol. 2011 Oct;23(5):564-72. doi: 10.1016/j.coi.2011.08.001. Epub 2011 Aug 22. Curr Opin Immunol. 2011. PMID: 21865020 Review.
Cited by
-
Cell-intrinsic innate immune control of West Nile virus infection.Trends Immunol. 2012 Oct;33(10):522-30. doi: 10.1016/j.it.2012.05.008. Epub 2012 Jun 20. Trends Immunol. 2012. PMID: 22726607 Free PMC article. Review.
-
Positive-strand RNA virus replication organelles at a glance.J Cell Sci. 2024 Sep 1;137(17):jcs262164. doi: 10.1242/jcs.262164. Epub 2024 Sep 10. J Cell Sci. 2024. PMID: 39254430 Free PMC article. Review.
-
Mitochondria in the regulation of innate and adaptive immunity.Immunity. 2015 Mar 17;42(3):406-17. doi: 10.1016/j.immuni.2015.02.002. Immunity. 2015. PMID: 25786173 Free PMC article. Review.
-
Hepatitis C Virus. Strategies to Evade Antiviral Responses.Future Virol. 2014;9(12):1061-1075. doi: 10.2217/fvl.14.89. Future Virol. 2014. PMID: 25983854 Free PMC article.
-
Focal adhesion kinase is a component of antiviral RIG-I-like receptor signaling.Cell Host Microbe. 2012 Feb 16;11(2):153-66. doi: 10.1016/j.chom.2012.01.008. Cell Host Microbe. 2012. PMID: 22341464 Free PMC article.
References
-
- Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. - PubMed
-
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell. 2005;122:669–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

