Flip-flop pharmacokinetics--delivering a reversal of disposition: challenges and opportunities during drug development
- PMID: 21837267
- PMCID: PMC3152312
- DOI: 10.4155/tde.11.19
Flip-flop pharmacokinetics--delivering a reversal of disposition: challenges and opportunities during drug development
Abstract
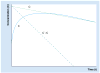
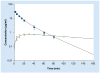
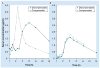
Flip-flop pharmacokinetics is a phenomenon often encountered with extravascularly administered drugs. Occurrence of flip-flop spans preclinical to human studies. The purpose of this article is to analyze both the pharmacokinetic interpretation errors and opportunities underlying the presence of flip-flop pharmacokinetics during drug development. Flip-flop occurs when the rate of absorption is slower than the rate of elimination. If it is not recognized, it can create difficulties in the acquisition and interpretation of pharmacokinetic parameters. When flip-flop is expected or discovered, a longer duration of sampling may be necessary in order to avoid overestimation of fraction of dose absorbed. Common culprits of flip-flop disposition are modified dosage formulations; however, formulation characteristics such as the drug chemical entities themselves or the incorporated excipients can also cause the phenomenon. Yet another contributing factor is the physiological makeup of the extravascular site of administration. In this article, these causes of flip-flop pharmacokinetics are discussed with incorporation of relevant examples and the implications for drug development outlined.
Figures




Similar articles
-
Few Drugs Display Flip-Flop Pharmacokinetics and These Are Primarily Associated with Classes 3 and 4 of the BDDCS.J Pharm Sci. 2015 Sep;104(9):3229-35. doi: 10.1002/jps.24505. Epub 2015 May 25. J Pharm Sci. 2015. PMID: 26010239 Free PMC article.
-
Slow drug delivery decreased total body clearance and altered bioavailability of immediate- and controlled-release oxycodone formulations.Pharmacol Res Perspect. 2016 Jan 22;4(1):e00210. doi: 10.1002/prp2.210. eCollection 2016 Feb. Pharmacol Res Perspect. 2016. PMID: 26977300 Free PMC article.
-
Evidence of a flip-flop phenomenon in acamprosate pharmacokinetics: an in vivo study in rats.Biopharm Drug Dispos. 2006 Oct;27(7):305-11. doi: 10.1002/bdd.513. Biopharm Drug Dispos. 2006. PMID: 16799924
-
Multiple peaking phenomena in pharmacokinetic disposition.Clin Pharmacokinet. 2010 Jun;49(6):351-77. doi: 10.2165/11319320-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20481648 Review.
-
Clinical pharmacokinetics of acamprosate.Clin Pharmacokinet. 1998 Nov;35(5):331-45. doi: 10.2165/00003088-199835050-00001. Clin Pharmacokinet. 1998. PMID: 9839087 Review.
Cited by
-
Upholding or Breaking the Law of Superposition in Pharmacokinetics.Biomedicines. 2024 Aug 13;12(8):1843. doi: 10.3390/biomedicines12081843. Biomedicines. 2024. PMID: 39200307 Free PMC article. Review.
-
Population Pharmacokinetic Modeling and Simulation for Dose Optimization of GB-5001, a Long-Acting Intramuscular Injection of Donepezil, in Healthy Participants.Neurol Ther. 2024 Oct;13(5):1453-1466. doi: 10.1007/s40120-024-00643-4. Epub 2024 Aug 10. Neurol Ther. 2024. PMID: 39126603 Free PMC article.
-
Enhancing acute inflammatory and sepsis treatment: superiority of membrane receptor blockade.Front Immunol. 2024 Jul 16;15:1424768. doi: 10.3389/fimmu.2024.1424768. eCollection 2024. Front Immunol. 2024. PMID: 39081318 Free PMC article. Review.
-
Comparable efficacy of oral bendamustine versus intravenous administration in treating hematologic malignancies.Cancer Chemother Pharmacol. 2024 Sep;94(3):361-372. doi: 10.1007/s00280-024-04688-y. Epub 2024 Jun 15. Cancer Chemother Pharmacol. 2024. PMID: 38878208
-
3D DLP-printed cannabinoid microneedles patch and its pharmacokinetic evaluation in rats.J Pharm Pharmacol. 2024 Jun 6;76(6):616-626. doi: 10.1093/jpp/rgae043. J Pharm Pharmacol. 2024. PMID: 38656627
References
Bibliography
-
- Zhou H. Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol. 2003;43(3):211–227. - PubMed
-
- Kaniwa N, Ogata H, Aoyagi N, Takeda Y, Uchiyama M. Power analyses of moment analysis parameter in bioequivalence tests. J Pharm Sci. 1989;78(12):1020–1024. - PubMed
-
- Toutain PL, Bousquet-Melou A. Plasma terminal half-life. J Vet Pharmacol Ther. 2004;27(6):427–439. - PubMed
-
- Byron PR, Notari RE. Critical analysis of “flip-flop” phenomenon in two-compartment pharmacokinetic model. J Pharm Sci. 1976;65(8):1140–1144. - PubMed
Websites
-
- US Department of Health and Human Services, FDA and Center for Veterinary Medicine (CVM) Guidance for Industry. Bioequivalence Guidance. 2006 November 8; www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/Gui....
-
- US Department of Health and Human Services, FDA and Center for Drug Evaluation and Research (CDER) Guidance for Industry. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products — General Considerations. 2003 March 19; www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guid....
-
- US Department of Health and Human Services, FDA and Center for Food Safety and Applied Nutrition. Guidance for Industry and Other Stakeholders Toxicological Principles for the Safety Assessment of Food Ingredients. 2007 July; www.fda.gov/downloads/Food/GuidanceComplianceRegulatoryInformation/Guida....
-
- Committee for Propietary Medicinal Products (CPMP) – European Medicines Agency. Guidance on Quality of Modified Release Products: A: Oral Dosage Forms B: Transdermal Dosage Forms. 1999 July 29; www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/....
-
- Committee for Medicinal Products for Human Use (CHMP) – European Medicines Agency. Guideline on the Investigation of Bioequivalence. 2010 January 20; www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
