Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell
- PMID: 21833583
- PMCID: PMC3173633
- DOI: 10.1007/s00018-011-0784-5
Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell
Abstract
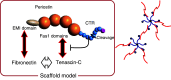
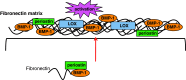
More than 10 years have passed since the naming of periostin derived from its expression sites in the periosteum and periodontal ligament. Following this finding, we have accumulated more data on the expression patterns of periostin, and, finally, with the generation of periostin-deficient mice, have revealed functions of periostin in the regeneration of tissues in bone, tooth, heart, and skin, and its action in cancer invasion. Since periostin is a matricellular protein, the first investigation of periostin function showed its enhancement of cell migration by acting outside the cell. On the other hand, recent observations have demonstrated that periostin functions in fibrillogenesis in association with extracellular matrix molecules inside the cell.
© The Author(s) 2011. This article is published with open access at Springerlink.com
Figures


Similar articles
-
Functions of Periostin in Dental Tissues and Its Role in Periodontal Tissue Regeneration.Adv Exp Med Biol. 2019;1132:63-72. doi: 10.1007/978-981-13-6657-4_7. Adv Exp Med Biol. 2019. PMID: 31037625 Review.
-
Functions of Periostin in dental tissues and its role in periodontal tissues' regeneration.Cell Mol Life Sci. 2017 Dec;74(23):4279-4286. doi: 10.1007/s00018-017-2645-3. Epub 2017 Sep 9. Cell Mol Life Sci. 2017. PMID: 28889194 Free PMC article. Review.
-
Altered distribution of extracellular matrix proteins in the periodontal ligament of periostin-deficient mice.Histol Histopathol. 2014 Jun;29(6):731-42. doi: 10.14670/HH-29.731. Epub 2013 Dec 19. Histol Histopathol. 2014. PMID: 24352874
-
PERIOSTIN: role in formation and maintenance of dental tissues.J Cell Physiol. 2014 Jan;229(1):1-5. doi: 10.1002/jcp.24407. J Cell Physiol. 2014. PMID: 23702840 Review.
-
Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture.J Biol Chem. 2010 Jan 15;285(3):2028-39. doi: 10.1074/jbc.M109.051961. Epub 2009 Nov 3. J Biol Chem. 2010. PMID: 19887451 Free PMC article.
Cited by
-
Periostin down-regulation attenuates the pro-fibrogenic response of hepatic stellate cells induced by TGF-β1.J Cell Mol Med. 2015 Oct;19(10):2462-8. doi: 10.1111/jcmm.12636. Epub 2015 Aug 7. J Cell Mol Med. 2015. PMID: 26249143 Free PMC article.
-
Prognostic significance of periostin in colorectal cancer.Chin J Cancer Res. 2019 Jun;31(3):547-556. doi: 10.21147/j.issn.1000-9604.2019.03.16. Chin J Cancer Res. 2019. PMID: 31354223 Free PMC article.
-
Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease.Kidney Int. 2014 Apr;85(4):845-54. doi: 10.1038/ki.2013.488. Epub 2013 Nov 27. Kidney Int. 2014. PMID: 24284511 Free PMC article.
-
Molecular biomarkers in idiopathic pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2014 Nov 1;307(9):L681-91. doi: 10.1152/ajplung.00014.2014. Epub 2014 Sep 26. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25260757 Free PMC article. Review.
-
Dynamic interplay between IL-1 and WNT pathways in regulating dermal adipocyte lineage cells during skin development and wound regeneration.Cell Rep. 2023 Jun 27;42(6):112647. doi: 10.1016/j.celrep.2023.112647. Epub 2023 Jun 16. Cell Rep. 2023. PMID: 37330908 Free PMC article.
References
-
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor β. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. - DOI - PubMed
-
- Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P, Kannicht C, Kzhyshkowska J, Longati P, Velten FW, Johansson S, Goerdt S. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J. 2002;362:155–164. doi: 10.1042/0264-6021:3620155. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

