Astrocytes from familial and sporadic ALS patients are toxic to motor neurons
- PMID: 21832997
- PMCID: PMC3170425
- DOI: 10.1038/nbt.1957
Astrocytes from familial and sporadic ALS patients are toxic to motor neurons
Abstract
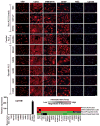
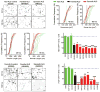
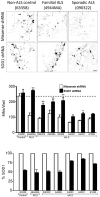
Amyotrophic lateral sclerosis (ALS) is a fatal motor neuron disease, with astrocytes implicated as contributing substantially to motor neuron death in familial (F)ALS. However, the proposed role of astrocytes in the pathology of ALS derives in part from rodent models of FALS based upon dominant mutations within the superoxide dismutase 1 (SOD1) gene, which account for <2% of all ALS cases. Their role in sporadic (S)ALS, which affects >90% of ALS patients, remains to be established. Using astrocytes generated from postmortem tissue from both FALS and SALS patients, we show that astrocytes derived from both patient groups are similarly toxic to motor neurons. We also demonstrate that SOD1 is a viable target for SALS, as its knockdown significantly attenuates astrocyte-mediated toxicity toward motor neurons. Our data highlight astrocytes as a non-cell autonomous component in SALS and provide an in vitro model system to investigate common disease mechanisms and evaluate potential therapies for SALS and FALS.
Figures

Similar articles
-
Necroptosis drives motor neuron death in models of both sporadic and familial ALS.Neuron. 2014 Mar 5;81(5):1001-1008. doi: 10.1016/j.neuron.2014.01.011. Epub 2014 Feb 6. Neuron. 2014. PMID: 24508385 Free PMC article.
-
Enhancing NAD+ Salvage Pathway Reverts the Toxicity of Primary Astrocytes Expressing Amyotrophic Lateral Sclerosis-linked Mutant Superoxide Dismutase 1 (SOD1).J Biol Chem. 2016 May 13;291(20):10836-46. doi: 10.1074/jbc.M115.698779. Epub 2016 Mar 21. J Biol Chem. 2016. PMID: 27002158 Free PMC article.
-
Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons.Nat Neurosci. 2007 May;10(5):615-22. doi: 10.1038/nn1876. Epub 2007 Apr 15. Nat Neurosci. 2007. PMID: 17435755 Free PMC article.
-
Rodent Models of Amyotrophic Lateral Sclerosis.Curr Protoc Pharmacol. 2015 Jun 1;69:5.67.1-5.67.21. doi: 10.1002/0471141755.ph0567s69. Curr Protoc Pharmacol. 2015. PMID: 26344214 Free PMC article. Review.
-
Recent advances in research on neuropathological aspects of familial amyotrophic lateral sclerosis with superoxide dismutase 1 gene mutations: neuronal Lewy body-like hyaline inclusions and astrocytic hyaline inclusions.Histol Histopathol. 1999 Jul;14(3):973-89. doi: 10.14670/HH-14.973. Histol Histopathol. 1999. PMID: 10425565 Review.
Cited by
-
Molecular chaperone Hsp110 rescues a vesicle transport defect produced by an ALS-associated mutant SOD1 protein in squid axoplasm.Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5428-33. doi: 10.1073/pnas.1303279110. Epub 2013 Mar 18. Proc Natl Acad Sci U S A. 2013. PMID: 23509252 Free PMC article.
-
Neuroprotective Effect of Bexarotene in the SOD1(G93A) Mouse Model of Amyotrophic Lateral Sclerosis.Front Cell Neurosci. 2015 Jul 1;9:250. doi: 10.3389/fncel.2015.00250. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26190974 Free PMC article.
-
Human Stem Cell-Derived Astrocytes: Specification and Relevance for Neurological Disorders.Curr Stem Cell Rep. 2016;2(3):236-247. doi: 10.1007/s40778-016-0049-1. Epub 2016 Jun 3. Curr Stem Cell Rep. 2016. PMID: 27547709 Free PMC article. Review.
-
Identification of Genetic Modifiers of TDP-43: Inflammatory Activation of Astrocytes for Neuroinflammation.Cells. 2021 Mar 18;10(3):676. doi: 10.3390/cells10030676. Cells. 2021. PMID: 33803845 Free PMC article.
-
Pathophysiological and diagnostic implications of cortical dysfunction in ALS.Nat Rev Neurol. 2016 Nov;12(11):651-661. doi: 10.1038/nrneurol.2016.140. Epub 2016 Sep 23. Nat Rev Neurol. 2016. PMID: 27658852 Review.
References
-
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. - PubMed
-
- Marchetto MC, et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 NS058224/NS/NINDS NIH HHS/United States
- R01 NS064492-03/NS/NINDS NIH HHS/United States
- F31NS058224/NS/NINDS NIH HHS/United States
- RC2 NS069476-02/NS/NINDS NIH HHS/United States
- R01 NS644912-1A1/NS/NINDS NIH HHS/United States
- R01 NS064492-01A1/NS/NINDS NIH HHS/United States
- R01 NS064492-02/NS/NINDS NIH HHS/United States
- RC2 NS069476-01/NS/NINDS NIH HHS/United States
- R01 NS064492/NS/NINDS NIH HHS/United States
- T32 NS077984/NS/NINDS NIH HHS/United States
- RC2 NS069476/NS/NINDS NIH HHS/United States
- RC2 NS69476-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

