Impaired alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking and function by mutant huntingtin
- PMID: 21832090
- PMCID: PMC3190808
- DOI: 10.1074/jbc.M111.236521
Impaired alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking and function by mutant huntingtin
Abstract
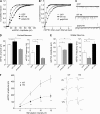
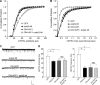
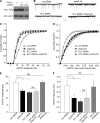
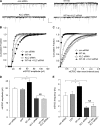
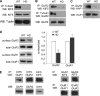
Emerging evidence from studies of Huntington disease (HD) pathophysiology suggests that huntingtin (htt) and its associated protein HAP1 participate in intracellular trafficking and synaptic function. However, it is largely unknown whether AMPA receptor trafficking, which is crucial for controlling the efficacy of synaptic excitation, is affected by the mutant huntingtin with polyglutamine expansion (polyQ-htt). In this study, we found that expressing polyQ-htt in neuronal cultures significantly decreased the amplitude and frequency of AMPAR-mediated miniature excitatory postsynaptic current (mEPSC), while expressing wild-type huntingtin (WT-htt) increased mEPSC. AMPAR-mediated synaptic transmission was also impaired in a transgenic mouse model of HD expressing polyQ-htt. The effect of polyQ-htt on mEPSC was mimicked by knockdown of HAP1 and occluded by the dominant negative HAP1. Moreover, we found that huntingtin affected mESPC via a mechanism depending on the kinesin motor protein, KIF5, which controls the transport of GluR2-containing AMPARs along microtubules in dendrites. The GluR2/KIF5/HAP1 complex was disrupted and dissociated from microtubules in the HD mouse model. Together, these data suggest that AMPAR trafficking and function is impaired by mutant huntingtin, presumably due to the interference of KIF5-mediated microtubule-based transport of AMPA receptors. The diminished strength of glutamatergic transmission could contribute to the deficits in movement control and cognitive processes in HD conditions.
Figures


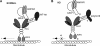
Similar articles
-
Disrupted GABAAR trafficking and synaptic inhibition in a mouse model of Huntington's disease.Neurobiol Dis. 2012 May;46(2):497-502. doi: 10.1016/j.nbd.2012.02.015. Epub 2012 Feb 28. Neurobiol Dis. 2012. PMID: 22402331 Free PMC article.
-
The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation.J Neurosci. 2014 Jan 22;34(4):1293-305. doi: 10.1523/JNEUROSCI.1870-13.2014. J Neurosci. 2014. PMID: 24453320 Free PMC article.
-
Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin.Neuron. 2010 Jan 14;65(1):53-65. doi: 10.1016/j.neuron.2009.12.007. Neuron. 2010. PMID: 20152113 Free PMC article.
-
[Huntington's disease: intracellular signaling pathways and neuronal death].J Soc Biol. 2005;199(3):247-51. doi: 10.1051/jbio:2005026. J Soc Biol. 2005. PMID: 16471265 Review. French.
-
Selective degeneration in YAC mouse models of Huntington disease.Brain Res Bull. 2007 Apr 30;72(2-3):124-31. doi: 10.1016/j.brainresbull.2006.10.018. Epub 2006 Nov 16. Brain Res Bull. 2007. PMID: 17352936 Review.
Cited by
-
Research advances in huntingtin-associated protein 1 and its application prospects in diseases.Front Neurosci. 2024 Jun 21;18:1402996. doi: 10.3389/fnins.2024.1402996. eCollection 2024. Front Neurosci. 2024. PMID: 38975245 Free PMC article. Review.
-
Selective synaptic targeting of the excitatory and inhibitory presynaptic organizers FGF22 and FGF7.J Cell Sci. 2015 Jan 15;128(2):281-92. doi: 10.1242/jcs.158337. Epub 2014 Nov 27. J Cell Sci. 2015. PMID: 25431136 Free PMC article.
-
Huntington's Disease: Mechanisms of Pathogenesis and Therapeutic Strategies.Cold Spring Harb Perspect Med. 2017 Jul 5;7(7):a024240. doi: 10.1101/cshperspect.a024240. Cold Spring Harb Perspect Med. 2017. PMID: 27940602 Free PMC article. Review.
-
Exosomes and Homeostatic Synaptic Plasticity Are Linked to Each other and to Huntington's, Parkinson's, and Other Neurodegenerative Diseases by Database-Enabled Analyses of Comprehensively Curated Datasets.Front Neurosci. 2017 Mar 31;11:149. doi: 10.3389/fnins.2017.00149. eCollection 2017. Front Neurosci. 2017. PMID: 28611571 Free PMC article.
-
Emerging Synaptic Molecules as Candidates in the Etiology of Neurological Disorders.Neural Plast. 2017;2017:8081758. doi: 10.1155/2017/8081758. Epub 2017 Feb 26. Neural Plast. 2017. PMID: 28331639 Free PMC article. Review.
References
-
- Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S. W., Bates G. P. (1996) Cell 87, 493–506 - PubMed
-
- Harjes P., Wanker E. E. (2003) Trends Biochem. Sci 28, 425–433 - PubMed
-
- Li X. J., Li S. H., Sharp A. H., Nucifora F. C., Jr., Schilling G., Lanahan A., Worley P., Snyder S. H., Ross C. A. (1995) Nature 378, 398–402 - PubMed
-
- Li X. J., Li S. H. (2005) Trends Pharmacol. Sci. 26, 1–3 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

