Hsc70 protein interaction with soluble and fibrillar alpha-synuclein
- PMID: 21832061
- PMCID: PMC3186418
- DOI: 10.1074/jbc.M111.261321
Hsc70 protein interaction with soluble and fibrillar alpha-synuclein
Abstract
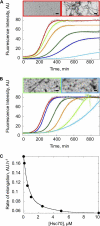
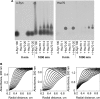
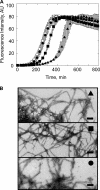
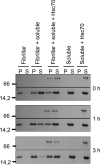
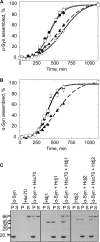
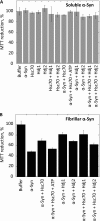
The aggregation of α-synuclein (α-Syn), the primary component of Lewy bodies, into high molecular weight assemblies is strongly associated with Parkinson disease. This event is believed to result from a conformational change within native α-Syn. Molecular chaperones exert critical housekeeping functions in vivo including refolding, maintaining in a soluble state, and/or pacifying protein aggregates. The influence of the stress-induced heat shock protein 70 (Hsp70) on α-Syn aggregation has been notably investigated. The constitutively expressed chaperone Hsc70 acts as an antiaggregation barrier before cells are overwhelmed with α-Syn aggregates and Hsp70 expression induced. Here, we investigate the interaction between Hsc70 and α-Syn, the consequences of this interaction, and the role of nucleotides and co-chaperones Hdj1 and Hdj2 as modulators. We show that Hsc70 sequesters soluble α-Syn in an assembly incompetent complex in the absence of ATP. The affinity of Hsc70 for soluble α-Syn diminishes upon addition of ATP alone or together with its co-chaperones Hdj1 or Hdj2 allowing faster binding and release of client proteins thus abolishing α-Syn assembly inhibition by Hsc70. We show that Hsc70 binds α-Syn fibrils with a 5-fold tighter affinity compared with soluble α-Syn. This suggests that Hsc70 preferentially interacts with high molecular weight α-Syn assemblies in vivo. Hsc70 binding certainly has an impact on the physicochemical properties of α-Syn assemblies. We show a reduced cellular toxicity of α-Syn fibrils coated with Hsc70 compared with "naked" fibrils. Hsc70 may therefore significantly affect the cellular propagation of α-Syn aggregates and their spread throughout the central nervous system in Parkinson disease.
Figures


Similar articles
-
Identification of protein interfaces between α-synuclein, the principal component of Lewy bodies in Parkinson disease, and the molecular chaperones human Hsc70 and the yeast Ssa1p.J Biol Chem. 2012 Sep 21;287(39):32630-9. doi: 10.1074/jbc.M112.387530. Epub 2012 Jul 26. J Biol Chem. 2012. PMID: 22843682 Free PMC article.
-
The C-terminal α-helices of mammalian Hsc70 play a critical role in the stabilization of α-synuclein binding and inhibition of aggregation.Int J Biol Macromol. 2016 Feb;83:433-41. doi: 10.1016/j.ijbiomac.2015.10.089. Epub 2015 Nov 19. Int J Biol Macromol. 2016. PMID: 26601760 Free PMC article.
-
Human Hsp70 Disaggregase Reverses Parkinson's-Linked α-Synuclein Amyloid Fibrils.Mol Cell. 2015 Sep 3;59(5):781-93. doi: 10.1016/j.molcel.2015.07.012. Epub 2015 Aug 20. Mol Cell. 2015. PMID: 26300264 Free PMC article.
-
Applying chaperones to protein-misfolding disorders: molecular chaperones against α-synuclein in Parkinson's disease.Int J Biol Macromol. 2013 Sep;60:196-205. doi: 10.1016/j.ijbiomac.2013.05.032. Epub 2013 Jun 5. Int J Biol Macromol. 2013. PMID: 23748003 Review.
-
Phase separation and other forms of α-Synuclein self-assemblies.Essays Biochem. 2022 Dec 16;66(7):987-1000. doi: 10.1042/EBC20220055. Essays Biochem. 2022. PMID: 36373662 Review.
Cited by
-
Polypeptides derived from α-Synuclein binding partners to prevent α-Synuclein fibrils interaction with and take-up by cells.PLoS One. 2020 Aug 13;15(8):e0237328. doi: 10.1371/journal.pone.0237328. eCollection 2020. PLoS One. 2020. PMID: 32790707 Free PMC article.
-
Targeting protein aggregation for the treatment of degenerative diseases.Nat Rev Drug Discov. 2015 Nov;14(11):759-80. doi: 10.1038/nrd4593. Epub 2015 Sep 4. Nat Rev Drug Discov. 2015. PMID: 26338154 Free PMC article. Review.
-
The interaction of Hsc70 protein with fibrillar α-Synuclein and its therapeutic potential in Parkinson's disease.Commun Integr Biol. 2012 Jan 1;5(1):94-5. doi: 10.4161/cib.18483. Commun Integr Biol. 2012. PMID: 22482021 Free PMC article.
-
Genetic Analysis of HSP40/DNAJ Family Genes in Parkinson's Disease: a Large Case-Control Study.Mol Neurobiol. 2022 Sep;59(9):5443-5451. doi: 10.1007/s12035-022-02920-5. Epub 2022 Jun 17. Mol Neurobiol. 2022. PMID: 35715682
-
Barcoding heat shock proteins to human diseases: looking beyond the heat shock response.Dis Model Mech. 2014 Apr;7(4):421-34. doi: 10.1242/dmm.014563. Dis Model Mech. 2014. PMID: 24719117 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

