Serological studies confirm the novel astrovirus HMOAstV-C as a highly prevalent human infectious agent
- PMID: 21829634
- PMCID: PMC3150362
- DOI: 10.1371/journal.pone.0022576
Serological studies confirm the novel astrovirus HMOAstV-C as a highly prevalent human infectious agent
Abstract
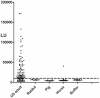
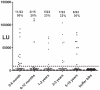
Molecular identification of a microbe is the first step in determining its prevalence of infection and pathogenic potential. Detection of specific adaptive immune responses can provide insights into whether a microbe is a human infectious agent and its epidemiology. Here we characterized human anti-IgG antibody responses by luciferase immunoprecipitation systems (LIPS) against two protein fragments derived from the capsid protein of the novel HMOAstV-C astrovirus. While antibodies to the N-terminal fragment were not informative, the C-terminal capsid fragment of HMOAstV-C showed a high frequency of immunoreactivity with serum from healthy blood donors. In contrast, a similar C-terminal capsid fragment from the related HMOAstV-A astrovirus failed to show immunoreactivity. Detailed analysis of adult serum from the United Sates using a standardized threshold demonstrated HMOAstV-C seropositivity in approximately 65% of the samples. Evaluation of serum samples from different pediatric age groups revealed that the prevalence of antibodies in 6-12 month, 1-2 year, 2-5 year and 5-10 year olds was 20%, 23%, 32% and 36%, respectively, indicating rising seroprevalence with age. Additionally, 50% (11/22) of the 0-6 month old children showed anti-HMOAstV-C antibody responses, likely reflecting maternal antibodies. Together these results document human humoral responses to HMOAstV-C and validate LIPS as a facile and effective approach for identifying humoral responses to novel infectious agents.
Conflict of interest statement
Figures


Similar articles
-
High Seropositivity Rate of Neutralizing Antibodies to Astrovirus VA1 in Human Populations.mSphere. 2021 Oct 27;6(5):e0048421. doi: 10.1128/mSphere.00484-21. Epub 2021 Sep 1. mSphere. 2021. PMID: 34468168 Free PMC article.
-
Seroepidemiology of astrovirus MLB1.Clin Vaccine Immunol. 2014 Jun;21(6):908-11. doi: 10.1128/CVI.00100-14. Epub 2014 Apr 30. Clin Vaccine Immunol. 2014. PMID: 24789796 Free PMC article.
-
[Antibody prevalence against astrovirus according to age groups].Kansenshogaku Zasshi. 1999 Jun;73(6):578-83. doi: 10.11150/kansenshogakuzasshi1970.73.578. Kansenshogaku Zasshi. 1999. PMID: 10423948 Japanese.
-
The changing epidemiology of astrovirus-associated gastroenteritis: a review.Arch Virol Suppl. 1996;12:287-300. doi: 10.1007/978-3-7091-6553-9_31. Arch Virol Suppl. 1996. PMID: 9015126 Review.
-
Role of astroviruses in childhood diarrhea.Curr Opin Pediatr. 2000 Jun;12(3):275-9. doi: 10.1097/00008480-200006000-00018. Curr Opin Pediatr. 2000. PMID: 10836166 Review.
Cited by
-
The Astrovirus Capsid: A Review.Viruses. 2017 Jan 19;9(1):15. doi: 10.3390/v9010015. Viruses. 2017. PMID: 28106836 Free PMC article. Review.
-
Comparison of novel MLB-clade, VA-clade and classic human astroviruses highlights constrained evolution of the classic human astrovirus nonstructural genes.Virology. 2013 Feb 5;436(1):8-14. doi: 10.1016/j.virol.2012.09.040. Epub 2012 Oct 16. Virology. 2013. PMID: 23084422 Free PMC article.
-
Antibody profiling of Borrelia burgdorferi infection in horses.Clin Vaccine Immunol. 2011 Sep;18(9):1562-7. doi: 10.1128/CVI.05123-11. Epub 2011 Jul 20. Clin Vaccine Immunol. 2011. PMID: 21775514 Free PMC article.
-
Identification of a second encephalitis-associated astrovirus in cattle.Emerg Microbes Infect. 2016 Jan 20;5(8):e71. doi: 10.1038/emi.2017.56. Emerg Microbes Infect. 2016. PMID: 26785943 Free PMC article. No abstract available.
-
Prevalence of classic, MLB-clade and VA-clade Astroviruses in Kenya and The Gambia.Virol J. 2015 May 15;12:78. doi: 10.1186/s12985-015-0299-z. Virol J. 2015. PMID: 25975198 Free PMC article.
References
-
- Guix S, Bosch A, Pinto RM. Human astrovirus diagnosis and typing: current and future prospects. Lett Appl Microbiol. 2005;41:103–105. - PubMed
-
- Jonassen CM, Jonassen TO, Saif YM, Snodgrass DR, Ushijima H, et al. Comparison of capsid sequences from human and animal astroviruses. J Gen Virol. 2001;82:1061–1067. - PubMed
-
- Jonassen CM, Jonassen TT, Sveen TM, Grinde B. Complete genomic sequences of astroviruses from sheep and turkey: comparison with related viruses. Virus Res. 2003;91:195–201. - PubMed
-
- Clark B, McKendrick M. A review of viral gastroenteritis. Curr Opin Infect Dis. 2004;17:461–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

