Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma
- PMID: 21829586
- PMCID: PMC3146530
- DOI: 10.1371/journal.pone.0023062
Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma
Abstract
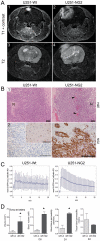
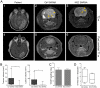
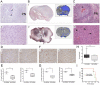
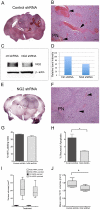
Aberrant expression of the progenitor marker Neuron-glia 2 (NG2/CSPG4) or melanoma proteoglycan on cancer cells and angiogenic vasculature is associated with an aggressive disease course in several malignancies including glioblastoma multiforme (GBM) and melanoma. Thus, we investigated the mechanism of NG2 mediated malignant progression and its potential as a therapeutic target in clinically relevant GBM and melanoma animal models. Xenografting NG2 overexpressing GBM cell lines resulted in increased growth rate, angiogenesis and vascular permeability compared to control, NG2 negative tumours. The effect of abrogating NG2 function was investigated after intracerebral delivery of lentivirally encoded shRNAs targeting NG2 in patient GBM xenografts as well as in established subcutaneous A375 melanoma tumours. NG2 knockdown reduced melanoma proliferation and increased apoptosis and necrosis. Targeting NG2 in two heterogeneous GBM xenografts significantly reduced tumour growth and oedema levels, angiogenesis and normalised vascular function. Vascular normalisation resulted in increased tumour invasion and decreased apoptosis and necrosis. We conclude that NG2 promotes tumour progression by multiple mechanisms and represents an amenable target for cancer molecular therapy.
Conflict of interest statement
Figures
Similar articles
-
A non-hierarchical organization of tumorigenic NG2 cells in glioblastoma promoted by EGFR.Neuro Oncol. 2019 Jun 10;21(6):719-729. doi: 10.1093/neuonc/noy204. Neuro Oncol. 2019. PMID: 30590711 Free PMC article.
-
Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival.Oncotarget. 2013 Sep;4(9):1527-46. doi: 10.18632/oncotarget.1291. Oncotarget. 2013. PMID: 24127551 Free PMC article.
-
On-target JAK2/STAT3 inhibition slows disease progression in orthotopic xenografts of human glioblastoma brain tumor stem cells.Neuro Oncol. 2013 Feb;15(2):198-207. doi: 10.1093/neuonc/nos302. Epub 2012 Dec 21. Neuro Oncol. 2013. PMID: 23262510 Free PMC article.
-
NG2 Proteoglycan-Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression.Microcirculation. 2016 Feb;23(2):122-33. doi: 10.1111/micc.12251. Microcirculation. 2016. PMID: 26465118 Free PMC article. Review.
-
NG2/HMP proteoglycan as a cancer therapeutic target.Methods Mol Biol. 2007;361:93-117. doi: 10.1385/1-59745-208-4:93. Methods Mol Biol. 2007. PMID: 17172708 Review.
Cited by
-
A Synopsis of Biomarkers in Glioblastoma: Past and Present.Curr Issues Mol Biol. 2024 Jul 3;46(7):6903-6939. doi: 10.3390/cimb46070412. Curr Issues Mol Biol. 2024. PMID: 39057054 Free PMC article. Review.
-
Oncofetal Chondroitin Sulfate Glycosaminoglycans Are Key Players in Integrin Signaling and Tumor Cell Motility.Mol Cancer Res. 2016 Dec;14(12):1288-1299. doi: 10.1158/1541-7786.MCR-16-0103. Epub 2016 Sep 21. Mol Cancer Res. 2016. PMID: 27655130 Free PMC article.
-
A non-hierarchical organization of tumorigenic NG2 cells in glioblastoma promoted by EGFR.Neuro Oncol. 2019 Jun 10;21(6):719-729. doi: 10.1093/neuonc/noy204. Neuro Oncol. 2019. PMID: 30590711 Free PMC article.
-
Bioprinting on Live Tissue for Investigating Cancer Cell Dynamics.Tissue Eng Part A. 2021 Apr;27(7-8):438-453. doi: 10.1089/ten.TEA.2020.0190. Epub 2020 Nov 18. Tissue Eng Part A. 2021. PMID: 33059528 Free PMC article.
-
CSPG4-Specific CAR.CIK Lymphocytes as a Novel Therapy for the Treatment of Multiple Soft-Tissue Sarcoma Histotypes.Clin Cancer Res. 2020 Dec 1;26(23):6321-6334. doi: 10.1158/1078-0432.CCR-20-0357. Epub 2020 Sep 8. Clin Cancer Res. 2020. PMID: 32900797 Free PMC article.
References
-
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. - PubMed
-
- Smith JS, Jenkins RB. Genetic alterations in adult diffuse glioma: occurrence, significance, and prognostic implications. Front Biosci. 2000;5:D213–231. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Behm FG, Smith FO, Raimondi SC, Pui CH, Bernstein ID. Human homologue of the rat chondroitin sulfate proteoglycan, NG2, detected by monoclonal antibody 7.1, identifies childhood acute lymphoblastic leukemias with t(4;11)(q21;q23) or t(11;19)(q23;p13) and MLL gene rearrangements. Blood. 1996;87:1134–1139. - PubMed
-
- Chekenya M, Enger PO, Thorsen F, Tysnes BB, Al-Sarraj S, et al. The glial precursor proteoglycan, NG2, is expressed on tumour neovasculature by vascular pericytes in human malignant brain tumours. Neuropathol Appl Neurobiol. 2002;28:367–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

