Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration
- PMID: 21828091
- PMCID: PMC3152921
- DOI: 10.1242/dev.064162
Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration
Abstract
Muscle regeneration requires the coordinated interaction of multiple cell types. Satellite cells have been implicated as the primary stem cell responsible for regenerating muscle, yet the necessity of these cells for regeneration has not been tested. Connective tissue fibroblasts also are likely to play a role in regeneration, as connective tissue fibrosis is a hallmark of regenerating muscle. However, the lack of molecular markers for these fibroblasts has precluded an investigation of their role. Using Tcf4, a newly identified fibroblast marker, and Pax7, a satellite cell marker, we found that after injury satellite cells and fibroblasts rapidly proliferate in close proximity to one another. To test the role of satellite cells and fibroblasts in muscle regeneration in vivo, we created Pax7(CreERT2) and Tcf4(CreERT2) mice and crossed these to R26R(DTA) mice to genetically ablate satellite cells and fibroblasts. Ablation of satellite cells resulted in a complete loss of regenerated muscle, as well as misregulation of fibroblasts and a dramatic increase in connective tissue. Ablation of fibroblasts altered the dynamics of satellite cells, leading to premature satellite cell differentiation, depletion of the early pool of satellite cells, and smaller regenerated myofibers. Thus, we provide direct, genetic evidence that satellite cells are required for muscle regeneration and also identify resident fibroblasts as a novel and vital component of the niche regulating satellite cell expansion during regeneration. Furthermore, we demonstrate that reciprocal interactions between fibroblasts and satellite cells contribute significantly to efficient, effective muscle regeneration.
Figures

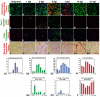

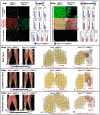
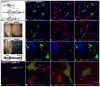
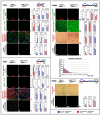
Similar articles
-
Connective tissue fibroblasts and Tcf4 regulate myogenesis.Development. 2011 Jan;138(2):371-84. doi: 10.1242/dev.057463. Development. 2011. PMID: 21177349 Free PMC article.
-
Brain and muscle Arnt-like 1 promotes skeletal muscle regeneration through satellite cell expansion.Exp Cell Res. 2015 Feb 1;331(1):200-210. doi: 10.1016/j.yexcr.2014.08.041. Epub 2014 Sep 9. Exp Cell Res. 2015. PMID: 25218946
-
Pax7 is critical for the normal function of satellite cells in adult skeletal muscle.Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16474-9. doi: 10.1073/pnas.1307680110. Epub 2013 Sep 24. Proc Natl Acad Sci U S A. 2013. PMID: 24065826 Free PMC article.
-
The molecular regulation of muscle stem cell function.Cold Spring Harb Symp Quant Biol. 2008;73:323-31. doi: 10.1101/sqb.2008.73.064. Epub 2009 Mar 27. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19329572 Review.
-
Concise Review: Epigenetic Regulation of Myogenesis in Health and Disease.Stem Cells Transl Med. 2016 Mar;5(3):282-90. doi: 10.5966/sctm.2015-0266. Epub 2016 Jan 21. Stem Cells Transl Med. 2016. PMID: 26798058 Free PMC article. Review.
Cited by
-
Intervertebral disc herniation effects on multifidus muscle composition and resident stem cell populations.JOR Spine. 2020 May 6;3(2):e1091. doi: 10.1002/jsp2.1091. eCollection 2020 Jun. JOR Spine. 2020. PMID: 32613166 Free PMC article.
-
Satellite cells and the muscle stem cell niche.Physiol Rev. 2013 Jan;93(1):23-67. doi: 10.1152/physrev.00043.2011. Physiol Rev. 2013. PMID: 23303905 Free PMC article. Review.
-
Tracing the developmental origin of tissue stem cells.Dev Growth Differ. 2022 Dec;64(9):566-576. doi: 10.1111/dgd.12816. Epub 2022 Nov 1. Dev Growth Differ. 2022. PMID: 36217609 Free PMC article. Review.
-
Myf5 expression during fetal myogenesis defines the developmental progenitors of adult satellite cells.Dev Biol. 2013 Jul 15;379(2):195-207. doi: 10.1016/j.ydbio.2013.04.021. Epub 2013 Apr 29. Dev Biol. 2013. PMID: 23639729 Free PMC article.
-
The altered fate of aging satellite cells is determined by signaling and epigenetic changes.Front Genet. 2015 Feb 20;6:59. doi: 10.3389/fgene.2015.00059. eCollection 2015. Front Genet. 2015. PMID: 25750654 Free PMC article.
References
-
- Alexakis C., Partridge T., Bou-Gharios G. (2007). Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am. J. Physiol. Cell Physiol. 293, C661-C669 - PubMed
-
- Bailey A. J., Shellswell G. B., Duance V. C. (1979). Identification and change of collagen types in differentiating myoblasts and developing chick muscle. Nature 278, 67-69 - PubMed
-
- Caldwell C. J., Mattey D. L., Weller R. O. (1990). Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol. Appl. Neurobiol. 16, 225-238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

