The cell biology of disease: cellular mechanisms of cardiomyopathy
- PMID: 21825071
- PMCID: PMC3153638
- DOI: 10.1083/jcb.201101100
The cell biology of disease: cellular mechanisms of cardiomyopathy
Abstract
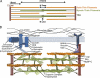
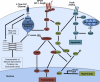
The heart exhibits remarkable adaptive responses to a wide array of genetic and extrinsic factors to maintain contractile function. When compensatory responses are not sustainable, cardiac dysfunction occurs, leading to cardiomyopathy. The many forms of cardiomyopathy exhibit a set of overlapping phenotypes reflecting the limited range of compensatory responses that the heart can use. These include cardiac hypertrophy, induction of genes normally expressed during development, fibrotic deposits that replace necrotic and apoptotic cardiomyocytes, and metabolic disturbances. The compensatory responses are mediated by signaling pathways that initially serve to maintain normal contractility; however, persistent activation of these pathways leads to cardiac dysfunction. Current research focuses on ways to target these specific pathways therapeutically.
Figures


Similar articles
-
Aldehyde dehydrogenase 2 ameliorates doxorubicin-induced myocardial dysfunction through detoxification of 4-HNE and suppression of autophagy.J Mol Cell Cardiol. 2014 Jun;71:92-104. doi: 10.1016/j.yjmcc.2014.01.002. Epub 2014 Jan 13. J Mol Cell Cardiol. 2014. PMID: 24434637
-
N-acetylcysteine abolishes the protective effect of losartan against left ventricular remodeling in cardiomyopathy hamster.Antioxid Redox Signal. 2008 Dec;10(12):1999-2008. doi: 10.1089/ars.2008.2069. Antioxid Redox Signal. 2008. PMID: 18665799
-
Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death.Mol Cell Biol. 2009 Jan;29(2):378-88. doi: 10.1128/MCB.01661-08. Epub 2008 Nov 10. Mol Cell Biol. 2009. PMID: 19001090 Free PMC article.
-
Cardiomyopathies and Related Changes in Contractility of Human Heart Muscle.Int J Mol Sci. 2018 Jul 31;19(8):2234. doi: 10.3390/ijms19082234. Int J Mol Sci. 2018. PMID: 30065175 Free PMC article. Review.
-
Metabolism in cardiomyopathy: every substrate matters.Cardiovasc Res. 2017 Mar 15;113(4):411-421. doi: 10.1093/cvr/cvx017. Cardiovasc Res. 2017. PMID: 28395011 Free PMC article. Review.
Cited by
-
Consequences of PDGFRα+ fibroblast reduction in adult murine hearts.Elife. 2022 Sep 23;11:e69854. doi: 10.7554/eLife.69854. Elife. 2022. PMID: 36149056 Free PMC article.
-
miR-218 Involvement in Cardiomyocyte Hypertrophy Is Likely through Targeting REST.Int J Mol Sci. 2016 May 31;17(6):848. doi: 10.3390/ijms17060848. Int J Mol Sci. 2016. PMID: 27258257 Free PMC article.
-
Body size and metabolic differences in Maine Coon cats with and without hypertrophic cardiomyopathy.J Feline Med Surg. 2013 Feb;15(2):74-80. doi: 10.1177/1098612X12460847. Epub 2012 Sep 21. J Feline Med Surg. 2013. PMID: 23001953 Free PMC article.
-
Molecular consequences of the R453C hypertrophic cardiomyopathy mutation on human β-cardiac myosin motor function.Proc Natl Acad Sci U S A. 2013 Jul 30;110(31):12607-12. doi: 10.1073/pnas.1309493110. Epub 2013 Jun 24. Proc Natl Acad Sci U S A. 2013. PMID: 23798412 Free PMC article.
-
Biology of the cardiac myocyte in heart disease.Mol Biol Cell. 2016 Jul 15;27(14):2149-60. doi: 10.1091/mbc.E16-01-0038. Mol Biol Cell. 2016. PMID: 27418636 Free PMC article. Review.
References
-
- Adams J.W., Migita D.S., Yu M.K., Young R., Hellickson M.S., Castro-Vargas F.E., Domingo J.D., Lee P.H., Bui J.S., Henderson S.A. 1996. Prostaglandin F2 alpha stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. J. Biol. Chem. 271:1179–1186 10.1074/jbc.271.2.1179 - DOI - PubMed
-
- Akyürek O., Akyürek N., Sayin T., Dinçer I., Berkalp B., Akyol G., Ozenci M., Oral D. 2001. Association between the severity of heart failure and the susceptibility of myocytes to apoptosis in patients with idiopathic dilated cardiomyopathy. Int. J. Cardiol. 80:29–36 10.1016/S0167-5273(01)00451-X - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

