HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway
- PMID: 21818118
- PMCID: PMC3263503
- DOI: 10.1038/cdd.2011.95
HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway
Abstract
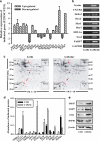
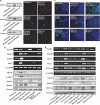
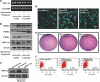
Hypoxia induces the expansion of glioblastoma stem cells (GSCs), but the mechanism underlying it is still unclear. Here, we supply evidence that hypoxia-inducible factor-1α (HIF-1α) induced activation of Notch pathway is essential for hypoxia-mediated maintenance of GSC. Either depletion of HIF-1α or inactivation of Notch signaling partly inhibits the hypoxia-mediated maintenance of GSC. Further data suggest a role for HIF-1α in the interaction and stabilization of intracellular domain of Notch (NICD), and activation of Notch signaling. The mRNA level of HIF-1α and Notch target gene FABP7 was elevated in GSC. And the STAT3 pathway responsible for the HIF-1α gene transcription, the phosphatidylinositol 3-kinase-Akt and ERK1/2, both of which are contributed to HIF-1α protein translation, are also preferentially activated in GSC. Inhibition of these pathways partly reduces the hypoxia-induced activation of the Notch pathway and subsequent GSC maintenance. Taken together, our findings suggest that HIF-1α requires Notch pathway to drive the maintenance of GSC. The activated regulation of HIF-1α makes GSC more sensitive to hypoxia-mediated maintenance. These findings enhance our understanding of mechanism of hypoxia-mediated GSC expansion and provide HIF-1α as an attractive target for glioblastoma therapy.
Figures





Similar articles
-
Hif-1α and Hif-2α differentially regulate Notch signaling through competitive interaction with the intracellular domain of Notch receptors in glioma stem cells.Cancer Lett. 2014 Jul 10;349(1):67-76. doi: 10.1016/j.canlet.2014.03.035. Epub 2014 Apr 3. Cancer Lett. 2014. PMID: 24705306
-
Cardiac glycosides suppress the maintenance of stemness and malignancy via inhibiting HIF-1α in human glioma stem cells.Oncotarget. 2017 Jun 20;8(25):40233-40245. doi: 10.18632/oncotarget.16714. Oncotarget. 2017. PMID: 28410215 Free PMC article.
-
HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways.J Exp Clin Cancer Res. 2018 Oct 19;37(1):256. doi: 10.1186/s13046-018-0925-x. J Exp Clin Cancer Res. 2018. PMID: 30340507 Free PMC article.
-
Effect of hypoxia-inducible factors in normal and leukemic stem cell regulation and their potential therapeutic impact.Expert Opin Biol Ther. 2016;16(4):463-76. doi: 10.1517/14712598.2016.1133582. Epub 2016 Jan 28. Expert Opin Biol Ther. 2016. PMID: 26679619 Review.
-
Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function (Review).Mol Med Rep. 2015 Aug;12(2):2411-6. doi: 10.3892/mmr.2015.3689. Epub 2015 Apr 27. Mol Med Rep. 2015. PMID: 25936862 Review.
Cited by
-
PFKFB4 interacts with FBXO28 to promote HIF-1α signaling in glioblastoma.Oncogenesis. 2022 Sep 17;11(1):57. doi: 10.1038/s41389-022-00433-3. Oncogenesis. 2022. PMID: 36115843 Free PMC article.
-
Notch signaling change in pulmonary vascular remodeling in rats with pulmonary hypertension and its implication for therapeutic intervention.PLoS One. 2012;7(12):e51514. doi: 10.1371/journal.pone.0051514. Epub 2012 Dec 12. PLoS One. 2012. PMID: 23251561 Free PMC article.
-
The updated biology of hypoxia-inducible factor.EMBO J. 2012 May 30;31(11):2448-60. doi: 10.1038/emboj.2012.125. Epub 2012 May 4. EMBO J. 2012. PMID: 22562152 Free PMC article. Review.
-
Hypoxia Regulates the Self-Renewal of Endometrial Mesenchymal Stromal/Stem-like Cells via Notch Signaling.Int J Mol Sci. 2022 Apr 21;23(9):4613. doi: 10.3390/ijms23094613. Int J Mol Sci. 2022. PMID: 35563003 Free PMC article.
-
Functional In Vitro Assessment of VEGFA/NOTCH2 Signaling Pathway and pRB Proteasomal Degradation and the Clinical Relevance of Mucolipin TRPML2 Overexpression in Glioblastoma Patients.Int J Mol Sci. 2022 Jan 8;23(2):688. doi: 10.3390/ijms23020688. Int J Mol Sci. 2022. PMID: 35054871 Free PMC article.
References
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
-
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. - PubMed
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. - PubMed
-
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev cell. 2005;9:617–628. - PubMed
-
- Chen HL, Pistollato F, Hoeppner DJ, Ni HT, McKay RD, Panchision DM. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

