Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis
- PMID: 21813772
- PMCID: PMC3187621
- DOI: 10.4049/jimmunol.1100804
Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis
Abstract
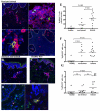
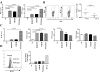
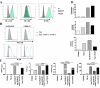
γδ T cells mediate rapid tissue responses in murine skin and participate in cutaneous immune regulation including protection against cancer. The role of human γδ cells in cutaneous homeostasis and pathology is characterized poorly. In this study, we show in vivo evidence that human blood contains a distinct subset of proinflammatory cutaneous lymphocyte Ag and CCR6-positive Vγ9Vδ2 T cells, which is rapidly recruited into perturbed human skin. Vγ9Vδ2 T cells produced an array of proinflammatory mediators including IL-17A and activated keratinocytes in a TNF-α- and IFN-γ-dependent manner. Examination of the common inflammatory skin disease psoriasis revealed a striking reduction of circulating Vγ9Vδ2 T cells in psoriasis patients compared with healthy controls and atopic dermatitis patients. Decreased numbers of circulating Vγ9Vδ2 T cells normalized after successful treatment with psoriasis-targeted therapy. Taken together with the increased presence of Vγ9Vδ2 T cells in psoriatic skin, these data indicate redistribution of Vγ9Vδ2 T cells from the blood to the skin compartment in psoriasis. In summary, we report a novel human proinflammatory γδ T cell involved in skin immune surveillance with immediate response characteristics and with potential clinical relevance in inflammatory skin disease.
Figures




Similar articles
-
Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis.J Immunol. 2000 Jun 15;164(12):6621-32. doi: 10.4049/jimmunol.164.12.6621. J Immunol. 2000. PMID: 10843722
-
IL-17-Secreting γδ T Cells Are Completely Dependent upon CCR6 for Homing to Inflamed Skin.J Immunol. 2017 Nov 1;199(9):3129-3136. doi: 10.4049/jimmunol.1700826. Epub 2017 Sep 29. J Immunol. 2017. PMID: 28972090
-
Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis.J Immunol. 2011 Nov 15;187(10):5026-31. doi: 10.4049/jimmunol.1101817. Epub 2011 Oct 7. J Immunol. 2011. PMID: 21984702
-
Dissecting the complexity of γδ T-cell subsets in skin homeostasis, inflammation, and malignancy.J Allergy Clin Immunol. 2021 Jun;147(6):2030-2042. doi: 10.1016/j.jaci.2020.11.023. Epub 2020 Nov 28. J Allergy Clin Immunol. 2021. PMID: 33259837 Review.
-
Innate T cell immunity to HIV-infection. Immunotherapy with phosphocarbohydrates, a novel strategy of immune intervention?Vaccine. 2002 May 6;20(15):1938-41. doi: 10.1016/s0264-410x(02)00070-1. Vaccine. 2002. PMID: 11983250 Review.
Cited by
-
Mechanisms underlying lineage commitment and plasticity of human γδ T cells.Cell Mol Immunol. 2013 Jan;10(1):30-4. doi: 10.1038/cmi.2012.42. Epub 2012 Oct 22. Cell Mol Immunol. 2013. PMID: 23085943 Free PMC article. Review.
-
The protective role of tissue-resident interleukin 17A-producing gamma delta T cells in Mycobacterium leprae infection.Front Immunol. 2022 Oct 26;13:961405. doi: 10.3389/fimmu.2022.961405. eCollection 2022. Front Immunol. 2022. PMID: 36389696 Free PMC article.
-
Identification of distinct functional thymic programming of fetal and pediatric human γδ thymocytes via single-cell analysis.Nat Commun. 2022 Oct 4;13(1):5842. doi: 10.1038/s41467-022-33488-2. Nat Commun. 2022. PMID: 36195611 Free PMC article.
-
γδ T cells in rheumatic diseases: from fundamental mechanisms to autoimmunity.Semin Immunopathol. 2019 Sep;41(5):595-605. doi: 10.1007/s00281-019-00752-5. Epub 2019 Sep 10. Semin Immunopathol. 2019. PMID: 31506867 Free PMC article. Review.
-
Composite tissue allotransplantation: opportunities and challenges.Cell Mol Immunol. 2019 Apr;16(4):343-349. doi: 10.1038/s41423-019-0215-3. Epub 2019 Mar 6. Cell Mol Immunol. 2019. PMID: 30842628 Free PMC article. Review.
References
-
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. - PubMed
-
- Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. - PubMed
-
- Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, Kaplan DH, Hayday AC, Girardi M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. - PubMed
-
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

