Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons
- PMID: 21801874
- PMCID: PMC3157205
- DOI: 10.1016/j.ajpath.2011.04.013
Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons
Abstract
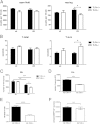
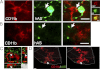
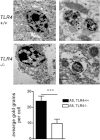
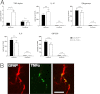
Toll-like receptors (TLRs) mediate innate immunity, and their dysregulation may play a role in α-synucleinopathies, such as Parkinson's disease or multiple system atrophy (MSA). The aim of this study was to define the role of TLR4 in α-synuclein-linked neurodegeneration. Ablation of TLR4 in a transgenic mouse model of MSA with oligodendroglial α-synuclein overexpression augmented motor disability and enhanced loss of nigrostriatal dopaminergic neurons. These changes were associated with increased brain levels of α-synuclein linked to disturbed TLR4-mediated microglial phagocytosis of α-synuclein. Furthermore, tumor necrosis factor-α levels were increased in the midbrain and associated with a proinflammatory astroglial response. Our data suggest that TLR4 ablation impairs the phagocytic response of microglia to α-synuclein and enhances neurodegeneration in a transgenic MSA mouse model. The study supports TLR4 signaling as innate neuroprotective mechanism acting through clearance of α-synuclein.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy.Mol Neurodegener. 2017 Jul 4;12(1):52. doi: 10.1186/s13024-017-0195-7. Mol Neurodegener. 2017. PMID: 28676095 Free PMC article.
-
The Potential Role of Toll-Like Receptor 4 in Mediating Dopaminergic Cell Loss and Alpha-Synuclein Expression in the Acute MPTP Mouse Model of Parkinson's Disease.J Mol Neurosci. 2018 Apr;64(4):611-618. doi: 10.1007/s12031-018-1057-7. Epub 2018 Mar 27. J Mol Neurosci. 2018. PMID: 29589201
-
Toll-like receptor 4 deficiency facilitates α-synuclein propagation and neurodegeneration in a mouse model of prodromal Parkinson's disease.Parkinsonism Relat Disord. 2021 Oct;91:59-65. doi: 10.1016/j.parkreldis.2021.09.007. Epub 2021 Sep 11. Parkinsonism Relat Disord. 2021. PMID: 34530328
-
The role of Toll-like receptors and neuroinflammation in Parkinson's disease.J Neuroinflammation. 2022 Jun 6;19(1):135. doi: 10.1186/s12974-022-02496-w. J Neuroinflammation. 2022. PMID: 35668422 Free PMC article. Review.
-
TLR2 and TLR4 in Parkinson's disease pathogenesis: the environment takes a toll on the gut.Transl Neurodegener. 2021 Nov 17;10(1):47. doi: 10.1186/s40035-021-00271-0. Transl Neurodegener. 2021. PMID: 34814947 Free PMC article. Review.
Cited by
-
Dual Roles of Microglia in the Basal Ganglia in Parkinson's Disease.Int J Mol Sci. 2021 Apr 9;22(8):3907. doi: 10.3390/ijms22083907. Int J Mol Sci. 2021. PMID: 33918947 Free PMC article. Review.
-
Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia.Glia. 2013 Mar;61(3):349-60. doi: 10.1002/glia.22437. Epub 2012 Oct 25. Glia. 2013. PMID: 23108585 Free PMC article.
-
Regulation of α-synuclein homeostasis and inflammasome activation by microglial autophagy.Sci Adv. 2022 Oct 28;8(43):eabn1298. doi: 10.1126/sciadv.abn1298. Epub 2022 Oct 26. Sci Adv. 2022. PMID: 36288297 Free PMC article. Review.
-
Lenalidomide reduces microglial activation and behavioral deficits in a transgenic model of Parkinson's disease.J Neuroinflammation. 2015 May 14;12:93. doi: 10.1186/s12974-015-0320-x. J Neuroinflammation. 2015. PMID: 25966683 Free PMC article.
-
Aspects of innate immunity and Parkinson's disease.Front Pharmacol. 2012 Mar 8;3:33. doi: 10.3389/fphar.2012.00033. eCollection 2012. Front Pharmacol. 2012. PMID: 22408621 Free PMC article.
References
-
- Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med. 2009;11:e22. - PubMed
-
- Al-Chalabi A., Durr A., Wood N.W., Parkinson M.H., Camuzat A., Hulot J.S., Morrison K.E., Renton A., Sussmuth S.D., Landwehrmeyer B.G., Ludolph A., Agid Y., Brice A., Leigh P.N., Bensimon G. Genetic variants of the alpha-synuclein gene SNCA are associated with multiple system atrophy. PLoS One. 2009;4:e7114. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS057096/NS/NINDS NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P30 NS057096/NS/NINDS NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- P01 NS044233/NS/NINDS NIH HHS/United States
- NS044233/NS/NINDS NIH HHS/United States
- P 19989/FWF_/Austrian Science Fund FWF/Austria
- AG022074/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
- F 4404/FWF_/Austrian Science Fund FWF/Austria
- AG5131/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

