Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics
- PMID: 21792905
- PMCID: PMC3130100
- DOI: 10.1002/jcp.22609
Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics
Abstract
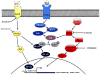
Glutamate is an essential excitatory neurotransmitter regulating brain functions. Excitatory amino acid transporter (EAAT)-2 is one of the major glutamate transporters expressed predominantly in astroglial cells and is responsible for 90% of total glutamate uptake. Glutamate transporters tightly regulate glutamate concentration in the synaptic cleft. Dysfunction of EAAT2 and accumulation of excessive extracellular glutamate has been implicated in the development of several neurodegenerative diseases including Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis. Analysis of the 2.5 kb human EAAT2 promoter showed that NF-κB is an important regulator of EAAT2 expression in astrocytes. Screening of approximately 1,040 FDA-approved compounds and nutritionals led to the discovery that many β-lactam antibiotics are transcriptional activators of EAAT2 resulting in increased EAAT2 protein levels. Treatment of animals with ceftriaxone (CEF), a β-lactam antibiotic, led to an increase of EAAT2 expression and glutamate transport activity in the brain. CEF has neuroprotective effects in both in vitro and in vivo models based on its ability to inhibit neuronal cell death by preventing glutamate excitotoxicity. CEF increases EAAT2 transcription in primary human fetal astrocytes through the NF-κB signaling pathway. The NF-κB binding site at -272 position was critical in CEF-mediated EAAT2 protein induction. These studies emphasize the importance of transcriptional regulation in controlling glutamate levels in the brain. They also emphasize the potential utility of the EAAT2 promoter for developing both low and high throughput screening assays to identify novel small molecule regulators of glutamate transport with potential to ameliorate pathological changes occurring during and causing neurodegeneration.
Copyright © 2010 Wiley-Liss, Inc.
Figures


Similar articles
-
Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes.J Biol Chem. 2008 May 9;283(19):13116-23. doi: 10.1074/jbc.M707697200. Epub 2008 Mar 7. J Biol Chem. 2008. PMID: 18326497 Free PMC article.
-
Harmine, a natural beta-carboline alkaloid, upregulates astroglial glutamate transporter expression.Neuropharmacology. 2011 Jun;60(7-8):1168-75. doi: 10.1016/j.neuropharm.2010.10.016. Epub 2010 Oct 27. Neuropharmacology. 2011. PMID: 21034752 Free PMC article.
-
Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2).Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1955-60. doi: 10.1073/pnas.0136555100. Epub 2003 Feb 10. Proc Natl Acad Sci U S A. 2003. PMID: 12578975 Free PMC article.
-
The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics.Neuropharmacology. 2019 Dec 15;161:107559. doi: 10.1016/j.neuropharm.2019.03.002. Epub 2019 Mar 6. Neuropharmacology. 2019. PMID: 30851309 Free PMC article. Review.
-
Role of glutamate excitotoxicity and glutamate transporter EAAT2 in epilepsy: Opportunities for novel therapeutics development.Biochem Pharmacol. 2021 Nov;193:114786. doi: 10.1016/j.bcp.2021.114786. Epub 2021 Sep 24. Biochem Pharmacol. 2021. PMID: 34571003 Free PMC article. Review.
Cited by
-
Neurobiology, Functions, and Relevance of Excitatory Amino Acid Transporters (EAATs) to Treatment of Refractory Epilepsy.CNS Drugs. 2020 Nov;34(11):1089-1103. doi: 10.1007/s40263-020-00764-y. CNS Drugs. 2020. PMID: 32926322 Review.
-
Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats.J Mol Neurosci. 2013 Nov;51(3):779-87. doi: 10.1007/s12031-013-0064-y. Epub 2013 Jul 27. J Mol Neurosci. 2013. PMID: 23893122 Free PMC article.
-
Development of mice with brain-specific deletion of floxed glud1 (glutamate dehydrogenase 1) using cre recombinase driven by the nestin promoter.Neurochem Res. 2014;39(3):456-9. doi: 10.1007/s11064-013-1041-0. Epub 2013 Apr 18. Neurochem Res. 2014. PMID: 23595828 Review.
-
Astroglia in Alzheimer's Disease.Adv Exp Med Biol. 2019;1175:273-324. doi: 10.1007/978-981-13-9913-8_11. Adv Exp Med Biol. 2019. PMID: 31583592 Free PMC article. Review.
-
Yokukansan, a Traditional Japanese Medicine, Enhances the Glutamate Transporter GLT-1 Function in Cultured Rat Cortical Astrocytes.Evid Based Complement Alternat Med. 2018 May 6;2018:6804017. doi: 10.1155/2018/6804017. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 29853967 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

