Innate immune responses to bacterial ligands in the peripheral human lung--role of alveolar epithelial TLR expression and signalling
- PMID: 21789185
- PMCID: PMC3137597
- DOI: 10.1371/journal.pone.0021827
Innate immune responses to bacterial ligands in the peripheral human lung--role of alveolar epithelial TLR expression and signalling
Abstract
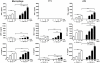
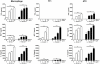
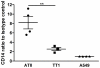
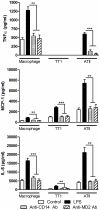
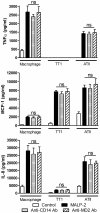
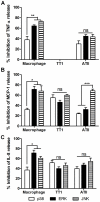
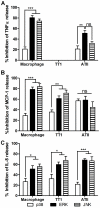
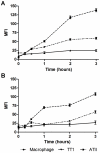
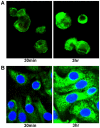
It is widely believed that the alveolar epithelium is unresponsive to LPS, in the absence of serum, due to low expression of TLR4 and CD14. Furthermore, the responsiveness of the epithelium to TLR-2 ligands is also poorly understood. We hypothesised that human alveolar type I (ATI) and type II (ATII) epithelial cells were responsive to TLR2 and TLR4 ligands (MALP-2 and LPS respectively), expressed the necessary TLRs and co-receptors (CD14 and MD2) and released distinct profiles of cytokines via differential activation of MAP kinases. Primary ATII cells and alveolar macrophages and an immortalised ATI cell line (TT1) elicited CD14 and MD2-dependent responses to LPS which did not require the addition of exogenous soluble CD14. TT1 and primary ATII cells expressed CD14 whereas A549 cells did not, as confirmed by flow cytometry. Following LPS and MALP-2 exposure, macrophages and ATII cells released significant amounts of TNFα, IL-8 and MCP-1 whereas TT1 cells only released IL-8 and MCP-1. P38, ERK and JNK were involved in MALP-2 and LPS-induced cytokine release from all three cell types. However, ERK and JNK were significantly more important than p38 in cytokine release from macrophages whereas all three were similarly involved in LPS-induced mediator release from TT1 cells. In ATII cells, JNK was significantly more important than p38 and ERK in LPS-induced MCP-1 release. MALP-2 and LPS exposure stimulated TLR4 protein expression in all three cell types; significantly more so in ATII cells than macrophages and TT1 cells. In conclusion, this is the first study describing the expression of CD14 on, and TLR2 and 4 signalling in, primary human ATII cells and ATI cells; suggesting that differential activation of MAP kinases, cytokine secretion and TLR4 expression by the alveolar epithelium and macrophages is important in orchestrating a co-ordinated response to inhaled pathogens.
Conflict of interest statement
Figures
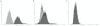


Similar articles
-
Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition.J Biol Chem. 2008 Sep 5;283(36):24748-59. doi: 10.1074/jbc.M800352200. Epub 2008 Jun 17. J Biol Chem. 2008. PMID: 18559343 Free PMC article.
-
Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages.J Immunol. 2007 Jan 1;178(1):463-73. doi: 10.4049/jimmunol.178.1.463. J Immunol. 2007. PMID: 17182585
-
Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists.J Immunol. 2007 Nov 1;179(9):6097-106. doi: 10.4049/jimmunol.179.9.6097. J Immunol. 2007. PMID: 17947684
-
[CD14 protein as a modulator of the inflammatory response].Postepy Biochem. 2024 Jan 30;69(4):274-282. doi: 10.18388/pb.2021_501. Print 2024 Jan 30. Postepy Biochem. 2024. PMID: 39012698 Review. Polish.
-
Mechanisms of ATII-to-ATI Cell Differentiation during Lung Regeneration.Int J Mol Sci. 2020 Apr 30;21(9):3188. doi: 10.3390/ijms21093188. Int J Mol Sci. 2020. PMID: 32366033 Free PMC article. Review.
Cited by
-
The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems.PLoS One. 2012;7(2):e32125. doi: 10.1371/journal.pone.0032125. Epub 2012 Feb 29. PLoS One. 2012. PMID: 22393384 Free PMC article.
-
Lung epithelial cells interact with immune cells and bacteria to shape the microenvironment in tuberculosis.Thorax. 2022 Apr;77(4):408-416. doi: 10.1136/thoraxjnl-2021-217997. Epub 2022 Jan 11. Thorax. 2022. PMID: 35017314 Free PMC article. Review.
-
Alarmin S100A8 Activates Alveolar Epithelial Cells in the Context of Acute Lung Injury in a TLR4-Dependent Manner.Front Immunol. 2017 Nov 13;8:1493. doi: 10.3389/fimmu.2017.01493. eCollection 2017. Front Immunol. 2017. PMID: 29180999 Free PMC article.
-
Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease.Front Microbiol. 2022 Oct 6;13:979719. doi: 10.3389/fmicb.2022.979719. eCollection 2022. Front Microbiol. 2022. PMID: 36274722 Free PMC article. Review.
-
Alveolar-Capillary Membrane-Related Pulmonary Cells as a Target in Endotoxin-Induced Acute Lung Injury.Int J Mol Sci. 2019 Feb 15;20(4):831. doi: 10.3390/ijms20040831. Int J Mol Sci. 2019. PMID: 30769918 Free PMC article. Review.
References
-
- Albiger B, Dahlberg S, Henriques-Normark B, Normark S. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med. 2007;261:511–528. - PubMed
-
- West AP, Koblansky AA, Ghosh S. Recognition and Signaling by Toll-Like Receptors. Annual Review of Cell and Developmental Biology. 2006;22:409–437. - PubMed
-
- Wyllie DH, Kiss-Toth E, Visintin A, Smith SC, Boussouf S, et al. Evidence for an Accessory Protein Function for Toll-Like Receptor 1 in Anti-Bacterial Responses. J Immunol. 2000;165:7125–7132. - PubMed
-
- Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

