A Role for PML in Innate Immunity
- PMID: 21779477
- PMCID: PMC3111006
- DOI: 10.1177/1947601911402682
A Role for PML in Innate Immunity
Abstract
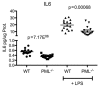
The promyelocytic leukemia gene (PML) of acute promyelocytic leukemia is an established tumor suppressor gene with critical functions in growth suppression, induction of apoptosis, and cellular senescence. Interestingly, although less studied, PML seems to play a key role also in immune response to viral infection. Herein, we report that Pml(-/-) mice spontaneously develop an atypical invasive and lethal granulomatous lesion known as botryomycosis (BTM). In Pml(-/-) mice, BTM is the result of impaired function of macrophages, whereby they fail to become activated and are thus unable to clear pathogenic microorganisms. Accordingly, Pml(-/-) mice are resistant to lipopolysaccharide (LPS)-induced septic shock as a result of an ineffective production of cytokines and chemokines, suggesting a role for PML in the innate immune Toll-like receptor (TLR)/NF-κB prosurvival pathway. These results not only shed light on a new fundamental function of PML in innate immunity, but they also point to a proto-oncogenic role for PML in certain cellular and pathological contexts.
Keywords: PML; botryomycosis; innate immunity.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures

Similar articles
-
A Tale of Usurpation and Subversion: SUMO-Dependent Integrity of Promyelocytic Leukemia Nuclear Bodies at the Crossroad of Infection and Immunity.Front Cell Dev Biol. 2021 Aug 27;9:696234. doi: 10.3389/fcell.2021.696234. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34513832 Free PMC article. Review.
-
Promyelocytic leukemia protein sensitizes tumor necrosis factor alpha-induced apoptosis by inhibiting the NF-kappaB survival pathway.J Biol Chem. 2003 Apr 4;278(14):12294-304. doi: 10.1074/jbc.M211849200. Epub 2003 Jan 22. J Biol Chem. 2003. PMID: 12540841
-
Characterization of Recombinant Human Cytomegaloviruses Encoding IE1 Mutants L174P and 1-382 Reveals that Viral Targeting of PML Bodies Perturbs both Intrinsic and Innate Immune Responses.J Virol. 2015 Nov 11;90(3):1190-205. doi: 10.1128/JVI.01973-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26559840 Free PMC article.
-
Translation-dependent mechanisms lead to PML upregulation and mediate oncogenic K-RAS-induced cellular senescence.EMBO Mol Med. 2012 Jul;4(7):594-602. doi: 10.1002/emmm.201200233. Epub 2012 Mar 21. EMBO Mol Med. 2012. PMID: 22359342 Free PMC article.
-
The molecular biology of acute promyelocytic leukemia.Cancer Treat Res. 1999;99:75-124. doi: 10.1007/978-0-585-38571-6_4. Cancer Treat Res. 1999. PMID: 9891864 Review.
Cited by
-
Behavior of Assembled Promyelocytic Leukemia Nuclear Bodies upon Asymmetric Division in Mouse Oocytes.Int J Mol Sci. 2024 Aug 8;25(16):8656. doi: 10.3390/ijms25168656. Int J Mol Sci. 2024. PMID: 39201340 Free PMC article.
-
Triple motif proteins 19 and 38 correlated with treatment responses and HBsAg clearance in HBeAg-negative chronic hepatitis B patients during peg-IFN-α therapy.Virol J. 2023 Jul 20;20(1):161. doi: 10.1186/s12985-023-02119-7. Virol J. 2023. PMID: 37475028 Free PMC article.
-
Promyelocytic Leukemia Protein (PML) Controls Listeria monocytogenes Infection.mBio. 2017 Jan 10;8(1):e02179-16. doi: 10.1128/mBio.02179-16. mBio. 2017. PMID: 28074026 Free PMC article.
-
Leishmania donovani chaperonin 10 regulates parasite internalization and intracellular survival in human macrophages.Med Microbiol Immunol. 2017 Jun;206(3):235-257. doi: 10.1007/s00430-017-0500-7. Epub 2017 Mar 11. Med Microbiol Immunol. 2017. PMID: 28283754
-
Emerging Role of PML Nuclear Bodies in Innate Immune Signaling.J Virol. 2016 Jun 10;90(13):5850-5854. doi: 10.1128/JVI.01979-15. Print 2016 Jul 1. J Virol. 2016. PMID: 27053550 Free PMC article. Review.
References
-
- Bernardi R, Papa A, Pandolfi PP. Regulation of apoptosis by PML and the PML-NBs. Oncogene. 2008;27:6299-312 - PubMed
-
- Salomoni P, Pandolfi PP. The role of PML in tumor suppression. Cell. 2002;108:165-70 - PubMed
-
- Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-beta signalling. Nature. 2004;431:205-11 - PubMed
-
- Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006-16 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
