Ablation of cellular prion protein does not ameliorate abnormal neural network activity or cognitive dysfunction in the J20 line of human amyloid precursor protein transgenic mice
- PMID: 21775587
- PMCID: PMC3314063
- DOI: 10.1523/JNEUROSCI.1459-11.2011
Ablation of cellular prion protein does not ameliorate abnormal neural network activity or cognitive dysfunction in the J20 line of human amyloid precursor protein transgenic mice
Erratum in
- J Neurosci. 2011 Sep 28;31(39):14046
Abstract
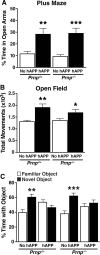
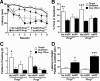
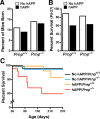
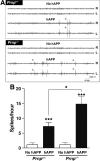
Previous studies suggested that the cellular prion protein (PrP(c)) plays a critical role in the pathogenesis of Alzheimer's disease (AD). Specifically, amyloid-β (Aβ) oligomers were proposed to cause synaptic and cognitive dysfunction by binding to PrP(c). To test this hypothesis, we crossed human amyloid precursor protein (hAPP) transgenic mice from line J20 onto a PrP(c)-deficient background. Ablation of PrP(c) did not prevent the premature mortality and abnormal neural network activity typically seen in hAPPJ20 mice. Furthermore, hAPPJ20 mice with or without PrP(c) expression showed comparably robust abnormalities in learning and memory and in other behavioral domains at 6-8 months of age. Notably, these abnormalities are not refractory to therapeutic manipulations in general: they can be effectively prevented by interventions that prevent Aβ-dependent neuronal dysfunction also in other lines of hAPP transgenic mice. Thus, at least in this model, PrP(c) is not an important mediator of Aβ-induced neurological impairments.
Figures
Similar articles
-
Amyloid-beta levels are significantly reduced and spatial memory defects are rescued in a novel neuroserpin-deficient Alzheimer's disease transgenic mouse model.J Neurochem. 2011 Sep;118(5):928-38. doi: 10.1111/j.1471-4159.2011.07359.x. Epub 2011 Jul 18. J Neurochem. 2011. PMID: 21689108
-
Suppression of Alzheimer's disease-related phenotypes by expression of heat shock protein 70 in mice.J Neurosci. 2011 Apr 6;31(14):5225-34. doi: 10.1523/JNEUROSCI.5478-10.2011. J Neurosci. 2011. PMID: 21471357 Free PMC article.
-
Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice.J Neurosci. 2009 Feb 18;29(7):1977-86. doi: 10.1523/JNEUROSCI.2984-08.2009. J Neurosci. 2009. PMID: 19228952 Free PMC article.
-
Memory impairment in transgenic Alzheimer mice requires cellular prion protein.J Neurosci. 2010 May 5;30(18):6367-74. doi: 10.1523/JNEUROSCI.0395-10.2010. J Neurosci. 2010. PMID: 20445063 Free PMC article.
-
Cognitive phenotyping of amyloid precursor protein transgenic J20 mice.Behav Brain Res. 2012 Mar 17;228(2):392-7. doi: 10.1016/j.bbr.2011.12.021. Epub 2011 Dec 19. Behav Brain Res. 2012. PMID: 22197298
Cited by
-
Characterization of AD-like phenotype in aged APPSwe/PS1dE9 mice.Age (Dordr). 2016 Aug;38(4):303-322. doi: 10.1007/s11357-016-9929-7. Epub 2016 Jul 21. Age (Dordr). 2016. PMID: 27439903 Free PMC article.
-
The neurodegeneration in Alzheimer disease and the prion protein.Prion. 2013 Jan-Feb;7(1):60-5. doi: 10.4161/pri.23286. Epub 2013 Jan 1. Prion. 2013. PMID: 23324596 Free PMC article. Review.
-
Aβ receptors specifically recognize molecular features displayed by fibril ends and neurotoxic oligomers.Nat Commun. 2021 Jun 8;12(1):3451. doi: 10.1038/s41467-021-23507-z. Nat Commun. 2021. PMID: 34103486 Free PMC article.
-
Amyloid beta precursor protein and prion protein have a conserved interaction affecting cell adhesion and CNS development.PLoS One. 2012;7(12):e51305. doi: 10.1371/journal.pone.0051305. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236467 Free PMC article.
-
Glutamate receptors function as scaffolds for the regulation of β-amyloid and cellular prion protein signaling complexes.Mol Brain. 2015 Mar 24;8:18. doi: 10.1186/s13041-015-0107-0. Mol Brain. 2015. PMID: 25888324 Free PMC article. Review.
References
-
- Aguzzi A, Baumann F, Bremer J. The prion's elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. - PubMed
-
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G. Synthetic amyloid-{beta} oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A. 2010;107:2295–2300. - PMC - PubMed
-
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H, DeArmond S, Prusiner S, Aguet M, Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG011385/AG/NIA NIH HHS/United States
- P01 AG022074-10/AG/NIA NIH HHS/United States
- R01 AG011385-08/AG/NIA NIH HHS/United States
- AG011385/AG/NIA NIH HHS/United States
- C06 RR018928-01/RR/NCRR NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
- NS065780/NS/NINDS NIH HHS/United States
- P30 NS065780-03/NS/NINDS NIH HHS/United States
- RR18928/RR/NCRR NIH HHS/United States
- P30 NS065780/NS/NINDS NIH HHS/United States
- R37 AG011385/AG/NIA NIH HHS/United States
- C06 RR018928/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
