PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor
- PMID: 21772253
- PMCID: PMC3242651
- DOI: 10.1038/mt.2011.131
PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor
Abstract
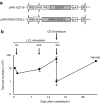
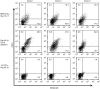
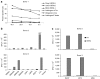
Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes (CTLs) can be modified to function as heterologous tumor directed effector cells that survive longer in vivo than tumor directed T cells without virus specificity, due to chronic stimulation by viral antigens expressed during persistent infection in seropositive individuals. We evaluated the nonviral piggyBac (PB) transposon system as a platform for modifying EBV-CTLs to express a functional human epidermal growth factor receptor 2-specific chimeric antigen receptor (HER2-CAR) thereby directing virus-specific, gene modified CTLs towards HER2-positive cancer cells. Peripheral blood mononuclear cells (PBMCs) were nucleofected with transposons encoding a HER2-CAR and a truncated CD19 molecule for selection followed by specific activation and expansion of EBV-CTLs. HER2-CAR was expressed in ~40% of T cells after CD19 selection with retention of immunophenotype, polyclonality, and function. HER2-CAR-modified EBV-CTLs (HER2-CTLs) killed HER2-positive brain tumor cell lines in vitro, exhibited transient and reversible increases in HER2-CAR expression following antigen-specific stimulation, and stably expressed HER2-CAR beyond 120 days. Adoptive transfer of PB-modified HER2-CTLs resulted in tumor regression in a murine xenograft model. Our results demonstrate that PB can be used to redirect virus-specific CTLs to tumor targets, which should prolong tumor-specific T cell survival in vivo producing more efficacious immunotherapy.
Figures





Similar articles
-
Treatment of advanced leukemia in mice with mRNA engineered T cells.Hum Gene Ther. 2011 Dec;22(12):1575-86. doi: 10.1089/hum.2011.070. Epub 2011 Sep 23. Hum Gene Ther. 2011. PMID: 21838572 Free PMC article.
-
Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies.Blood. 2003 Mar 1;101(5):1905-12. doi: 10.1182/blood-2002-05-1514. Epub 2002 Oct 31. Blood. 2003. PMID: 12411306
-
Target antigen expression on a professional antigen-presenting cell induces superior proliferative antitumor T-cell responses via chimeric T-cell receptors.J Immunother. 2006 Jan-Feb;29(1):21-31. doi: 10.1097/01.cji.0000175492.28723.d6. J Immunother. 2006. PMID: 16365597
-
Allogeneic chimeric antigen receptor-modified cells for adoptive cell therapy of cancer.Expert Opin Biol Ther. 2014 Jul;14(7):947-54. doi: 10.1517/14712598.2014.900540. Epub 2014 Mar 24. Expert Opin Biol Ther. 2014. PMID: 24661086 Review.
-
Targeting of tumor cells by lymphocytes engineered to express chimeric receptor genes.Cancer Immunol Immunother. 2004 Oct;53(10):893-903. doi: 10.1007/s00262-004-0523-y. Epub 2004 May 26. Cancer Immunol Immunother. 2004. PMID: 15168086 Free PMC article. Review.
Cited by
-
Lymphocyte expansion in bioreactors: upgrading adoptive cell therapy.J Biol Eng. 2021 Apr 13;15(1):13. doi: 10.1186/s13036-021-00264-7. J Biol Eng. 2021. PMID: 33849630 Free PMC article. Review.
-
Identification and selective expansion of functionally superior T cells expressing chimeric antigen receptors.J Transl Med. 2015 May 20;13:161. doi: 10.1186/s12967-015-0519-8. J Transl Med. 2015. PMID: 25990251 Free PMC article.
-
Generation of CAR T-cells using γ-retroviral vector.Methods Cell Biol. 2022;167:171-183. doi: 10.1016/bs.mcb.2021.06.014. Epub 2021 Jul 30. Methods Cell Biol. 2022. PMID: 35152995 Free PMC article.
-
CAR T cell therapy for breast cancer: harnessing the tumor milieu to drive T cell activation.J Immunother Cancer. 2018 May 10;6(1):34. doi: 10.1186/s40425-018-0347-5. J Immunother Cancer. 2018. PMID: 29747685 Free PMC article.
-
Design and development of therapies using chimeric antigen receptor-expressing T cells.Immunol Rev. 2014 Jan;257(1):107-26. doi: 10.1111/imr.12131. Immunol Rev. 2014. PMID: 24329793 Free PMC article. Review.
References
-
- Zendman AJ, Ruiter DJ., and, Van Muijen GN. Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol. 2003;194:272–288. - PubMed
-
- Eshhar Z, Waks T, Gross G., and, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. - PMC - PubMed
-
- Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM., and, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. - PubMed
-
- Finney HM, Akbar AN., and, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

