Therapeutic strategies to enhance the anticancer efficacy of histone deacetylase inhibitors
- PMID: 21765634
- PMCID: PMC3134392
- DOI: 10.1155/2011/514261
Therapeutic strategies to enhance the anticancer efficacy of histone deacetylase inhibitors
Abstract
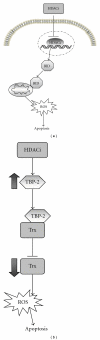
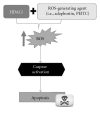
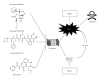
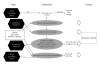
Histone acetylation is a posttranslational modification that plays a role in regulating gene expression. More recently, other nonhistone proteins have been identified to be acetylated which can regulate their function, stability, localization, or interaction with other molecules. Modulating acetylation with histone deacetylase inhibitors (HDACi) has been validated to have anticancer effects in preclinical and clinical cancer models. This has led to development and approval of the first HDACi, vorinostat, for the treatment of cutaneous T cell lymphoma. However, to date, targeting acetylation with HDACi as a monotherapy has shown modest activity against other cancers. To improve their efficacy, HDACi have been paired with other antitumor agents. Here, we discuss several combination therapies, highlighting various epigenetic drugs, ROS-generating agents, proteasome inhibitors, and DNA-damaging compounds that together may provide a therapeutic advantage over single-agent strategies.
Figures
Similar articles
-
Epigenetic therapy of cancer with histone deacetylase inhibitors.J Cancer Res Ther. 2014 Jul-Sep;10(3):469-78. doi: 10.4103/0973-1482.137937. J Cancer Res Ther. 2014. PMID: 25313724 Review.
-
Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy.Nutrients. 2018 Jun 6;10(6):731. doi: 10.3390/nu10060731. Nutrients. 2018. PMID: 29882797 Free PMC article. Review.
-
Histone deacetylases and epigenetic therapies of hematological malignancies.Pharmacol Res. 2010 Jul;62(1):18-34. doi: 10.1016/j.phrs.2010.02.010. Epub 2010 Feb 26. Pharmacol Res. 2010. PMID: 20219679 Review.
-
Targeting histone deacetylases in T-cell lymphoma.Leuk Lymphoma. 2017 Jun;58(6):1306-1319. doi: 10.1080/10428194.2016.1247956. Epub 2016 Nov 4. Leuk Lymphoma. 2017. PMID: 27813438 Review.
-
Histone Deacetylase Inhibitors as Anticancer Drugs.Int J Mol Sci. 2017 Jul 1;18(7):1414. doi: 10.3390/ijms18071414. Int J Mol Sci. 2017. PMID: 28671573 Free PMC article. Review.
Cited by
-
Endoplasmic reticulum stress plays a pivotal role in cell death mediated by the pan-deacetylase inhibitor panobinostat in human hepatocellular cancer cells.Transl Oncol. 2013 Apr;6(2):143-57. doi: 10.1593/tlo.12271. Epub 2013 Apr 1. Transl Oncol. 2013. PMID: 23544167 Free PMC article.
-
Role of Hydroxamate-Based Histone Deacetylase Inhibitors (Hb-HDACIs) in the Treatment of Solid Malignancies.Cancers (Basel). 2013 Jul 25;5(3):919-42. doi: 10.3390/cancers5030919. Cancers (Basel). 2013. PMID: 24202327 Free PMC article.
-
N4-acetylcytidine acetylation of neurexin 2 in the spinal dorsal horn regulates hypersensitivity in a rat model of cancer-induced bone pain.Mol Ther Nucleic Acids. 2024 Apr 23;35(2):102200. doi: 10.1016/j.omtn.2024.102200. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38831898 Free PMC article.
-
Regulators of gene expression as biomarkers for prostate cancer.Am J Cancer Res. 2012;2(6):620-57. Epub 2012 Nov 20. Am J Cancer Res. 2012. PMID: 23226612 Free PMC article.
-
Combination of bendamustine and entinostat synergistically inhibits proliferation of multiple myeloma cells via induction of apoptosis and DNA damage response.Cancer Lett. 2013 Jul 28;335(2):343-50. doi: 10.1016/j.canlet.2013.02.046. Epub 2013 Feb 28. Cancer Lett. 2013. PMID: 23459296 Free PMC article.
References
-
- Hong L, Schroth GP, Matthews HR, Yau P, Bradbury EM. Studies of the DNA binding properties of histone H4 amino terminus. Thermal denaturation studies reveal that acetylation markedly reduces the binding constant of the H4 ’tail’ to DNA. Journal of Biological Chemistry. 1993;268(1):305–314. - PubMed
-
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. - PubMed
-
- Gao L, Cueto MA, Asselbergs F, Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. Journal of Biological Chemistry. 2002;277(28):25748–25755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

