Role of SNX16 in the dynamics of tubulo-cisternal membrane domains of late endosomes
- PMID: 21754999
- PMCID: PMC3130770
- DOI: 10.1371/journal.pone.0021771
Role of SNX16 in the dynamics of tubulo-cisternal membrane domains of late endosomes
Abstract
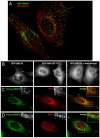
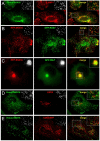
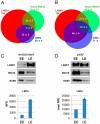
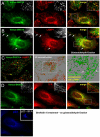
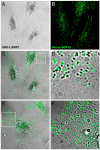
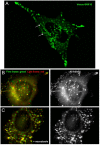
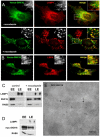
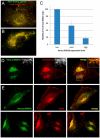
In this paper, we report that the PX domain-containing protein SNX16, a member of the sorting nexin family, is associated with late endosome membranes. We find that SNX16 is selectively enriched on tubulo-cisternal elements of this membrane system, whose highly dynamic properties and formation depend on intact microtubules. By contrast, SNX16 was not found on vacuolar elements that typically contain LBPA, and thus presumably correspond to multivesicular endosomes. We conclude that SNX16, together with its partner phosphoinositide, define a highly dynamic subset of late endosomal membranes, supporting the notion that late endosomes are organized in distinct morphological and functional regions. Our data also indicate that SNX16 is involved in tubule formation and cholesterol transport as well as trafficking of the tetraspanin CD81, suggesting that the protein plays a role in the regulation of late endosome membrane dynamics.
Conflict of interest statement
Figures
Similar articles
-
Evidence for a role of SNX16 in regulating traffic between the early and later endosomal compartments.J Biol Chem. 2003 Sep 5;278(36):34617-30. doi: 10.1074/jbc.M300143200. Epub 2003 Jun 17. J Biol Chem. 2003. PMID: 12813048
-
Sorting nexin 16 regulates EGF receptor trafficking by phosphatidylinositol-3-phosphate interaction with the Phox domain.J Cell Sci. 2004 Aug 15;117(Pt 18):4209-18. doi: 10.1242/jcs.01233. Epub 2004 Aug 3. J Cell Sci. 2004. PMID: 15292396
-
[Sorting Nexin 16:Structure,Function,and Role in Diseases].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022 Dec;44(6):1107-1111. doi: 10.3881/j.issn.1000-503X.14468. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022. PMID: 36373636 Review. Chinese.
-
SNX16 Regulates the Recycling of E-Cadherin through a Unique Mechanism of Coordinated Membrane and Cargo Binding.Structure. 2017 Aug 1;25(8):1251-1263.e5. doi: 10.1016/j.str.2017.06.015. Epub 2017 Jul 14. Structure. 2017. PMID: 28712807
-
SNX-PXA-RGS-PXC Subfamily of SNXs in the Regulation of Receptor-Mediated Signaling and Membrane Trafficking.Int J Mol Sci. 2021 Feb 26;22(5):2319. doi: 10.3390/ijms22052319. Int J Mol Sci. 2021. PMID: 33652569 Free PMC article. Review.
Cited by
-
Higher-order assembly of Sorting Nexin 16 controls tubulation and distribution of neuronal endosomes.J Cell Biol. 2019 Aug 5;218(8):2600-2618. doi: 10.1083/jcb.201811074. Epub 2019 Jun 28. J Cell Biol. 2019. PMID: 31253649 Free PMC article.
-
A Single Amino Acid Residue R144 of SNX16 Affects Its Ability to Inhibit the Replication of Influenza A Virus.Viruses. 2022 Apr 15;14(4):825. doi: 10.3390/v14040825. Viruses. 2022. PMID: 35458555 Free PMC article.
-
Parkin Modulates Endosomal Organization and Function of the Endo-Lysosomal Pathway.J Neurosci. 2016 Feb 24;36(8):2425-37. doi: 10.1523/JNEUROSCI.2569-15.2016. J Neurosci. 2016. PMID: 26911690 Free PMC article.
-
Normal dynactin complex function during synapse growth in Drosophila requires membrane binding by Arfaptin.Mol Biol Cell. 2013 Jun;24(11):1749-64, S1-5. doi: 10.1091/mbc.E12-09-0697. Epub 2013 Apr 17. Mol Biol Cell. 2013. PMID: 23596322 Free PMC article.
-
Circulating Anti-Sorting Nexins 16 Antibodies as an Emerging Biomarker of Coronary Artery Disease in Patients with Obstructive Sleep Apnea.Diagnostics (Basel). 2020 Jan 27;10(2):71. doi: 10.3390/diagnostics10020071. Diagnostics (Basel). 2020. PMID: 32012743 Free PMC article.
References
-
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. - PubMed
-
- Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. - PubMed
-
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. - PubMed
-
- Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006;119:605–614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

