Synthesis and evaluation of an anti-MLC1 × anti-CD90 bispecific antibody for targeting and retaining bone-marrow-derived multipotent stromal cells in infarcted myocardium
- PMID: 21749133
- PMCID: PMC3250066
- DOI: 10.1021/bc200309h
Synthesis and evaluation of an anti-MLC1 × anti-CD90 bispecific antibody for targeting and retaining bone-marrow-derived multipotent stromal cells in infarcted myocardium
Abstract
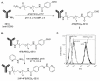
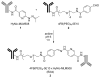
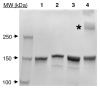
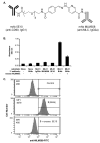
A key issue regarding the use of stem cells in cardiovascular regenerative medicine is their retention in target tissues. Here, we have generated and assessed a bispecific antibody heterodimer designed to improve the retention of bone-marrow-derived multipotent stromal cells (BMMSC) in cardiac tissue damaged by myocardial infarction. The heterodimer comprises an anti-human CD90 monoclonal antibody (mAb) (clone 5E10) and an anti-myosin light chain 1 (MLC1) mAb (clone MLM508) covalently cross-linked by a bis-arylhydrazone. We modified the anti-CD90 antibody with a pegylated-4-formylbenzamide moiety to a molar substitution ratio (MSR) of 2.6 and the anti-MLC1 antibody with a 6-hydrazinonicotinamide moiety to a MSR of 0.9. The covalent modifications had no significant deleterious effect on mAb epitope binding. Furthermore, the binding of anti-CD90 antibody to BMMSCs did not prevent their differentiation into adipo-, chondro-, or osteogenic lineages. Modified antibodies were combined under mild conditions (room temperature, pH 6, 1 h) in the presence of a catalyst (aniline) to allow for rapid generation of the covalent bis-arylhydrazone, which was monitored at A(354). We evaluated epitope immunoreactivity for each mAb in the construct. Flow cytometry demonstrated binding of the bispecific construct to BMMSCs that was competed by free anti-CD90 mAb, verifying that modification and cross-linking were not detrimental to the anti-CD90 complementarity-determining region. Similarly, ELISA-based assays demonstrated bispecific antibody binding to plastic-immobilized recombinant MLC1. Excess anti-MLC1 mAb competed for bispecific antibody binding. Finally, the anti-CD90 × anti-MLC1 bispecific antibody construct induced BMMSC adhesion to plastic-immobilized MLC1 that was resistant to shear stress, as measured in parallel-plate flow chamber assays. We used mAbs that bind both human antigens and the respective pig homologues. Thus, the anti-CD90 × anti-MLC1 bispecific antibody may be used in large animal studies of acute myocardial infarction and may provide a starting point for clinical studies.
Figures




Similar articles
-
Targeting human CD34+ hematopoietic stem cells with anti-CD45 x anti-myosin light-chain bispecific antibody preserves cardiac function in myocardial infarction.J Appl Physiol (1985). 2008 Jun;104(6):1793-800. doi: 10.1152/japplphysiol.01109.2007. Epub 2008 Feb 21. J Appl Physiol (1985). 2008. PMID: 18292296 Free PMC article.
-
Bispecific antibodies, nanoparticles and cells: bringing the right cells to get the job done.Expert Opin Biol Ther. 2015;15(9):1251-5. doi: 10.1517/14712598.2015.1049944. Epub 2015 May 25. Expert Opin Biol Ther. 2015. PMID: 26004388 Free PMC article.
-
Antibody targeting of stem cells to infarcted myocardium.Stem Cells. 2007 Mar;25(3):712-7. doi: 10.1634/stemcells.2005-0602. Epub 2006 Nov 30. Stem Cells. 2007. PMID: 17138964
-
Using bispecific antibodies in forced degradation studies to analyze the structure-function relationships of symmetrically and asymmetrically modified antibodies.MAbs. 2019 Aug/Sep;11(6):1101-1112. doi: 10.1080/19420862.2019.1618675. Epub 2019 Jun 4. MAbs. 2019. PMID: 31161859 Free PMC article. Review.
-
The new face of bispecific antibodies: targeting cancer and much more.Exp Hematol. 2006 Jan;34(1):1-6. doi: 10.1016/j.exphem.2005.07.013. Exp Hematol. 2006. PMID: 16413384 Review.
Cited by
-
Understanding and targeting cancer stem cells: therapeutic implications and challenges.Acta Pharmacol Sin. 2013 Jun;34(6):732-40. doi: 10.1038/aps.2013.27. Epub 2013 May 20. Acta Pharmacol Sin. 2013. PMID: 23685952 Free PMC article. Review.
-
Arming Mesenchymal Stromal/Stem Cells Against Cancer: Has the Time Come?Front Pharmacol. 2020 Sep 29;11:529921. doi: 10.3389/fphar.2020.529921. eCollection 2020. Front Pharmacol. 2020. PMID: 33117154 Free PMC article. Review.
-
MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation.Stem Cells Int. 2013;2013:732742. doi: 10.1155/2013/732742. Epub 2013 Aug 13. Stem Cells Int. 2013. PMID: 24000286 Free PMC article.
-
Targeted Therapy in Cardiovascular Disease: A Precision Therapy Era.Front Pharmacol. 2021 Apr 16;12:623674. doi: 10.3389/fphar.2021.623674. eCollection 2021. Front Pharmacol. 2021. PMID: 33935716 Free PMC article. Review.
-
Pharmacokinetic characteristics of mesenchymal stem cells in translational challenges.Signal Transduct Target Ther. 2024 Sep 13;9(1):242. doi: 10.1038/s41392-024-01936-8. Signal Transduct Target Ther. 2024. PMID: 39271680 Free PMC article. Review.
References
-
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch. Intern. Med. 2007;167:989–97. - PubMed
-
- Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J. Am. Coll. Cardiol. 2007;50:1761–7. - PubMed
-
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–902. - PubMed
-
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8:726–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

