Spry1 and Spry2 are necessary for lens vesicle separation and corneal differentiation
- PMID: 21743007
- PMCID: PMC3176024
- DOI: 10.1167/iovs.11-7531
Spry1 and Spry2 are necessary for lens vesicle separation and corneal differentiation
Abstract
Purpose: The studies reported here were performed to analyze the roles of Sproutys (Sprys), downstream targets and negative feedback regulators of the fibroblast growth factor (FGF) signaling pathway, in lens and corneal differentiation.
Methods: Spry1 and -2 were conditionally deleted in the lens and corneal epithelial precursors using the Le-Cre transgene and floxed alleles of Spry1 and -2. Alterations in lens and corneal development were assessed by hematoxylin and eosin staining, in situ hybridization, and immunohistochemistry.
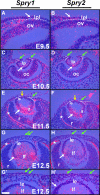
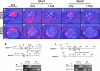
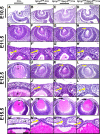
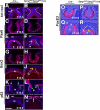
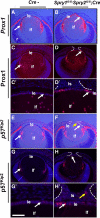
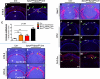
Results: Spry1 and -2 were upregulated in the lens fibers at the onset of fiber differentiation. FGF signaling was both necessary and sufficient for induction of Spry1 and -2 in the lens fiber cells. Spry1 and -2 single- or double-null lenses failed to separate from the overlying ectoderm and showed persistent keratolenticular stalks. Apoptosis of stalk cells, normally seen during lens vesicle detachment from the ectoderm, was inhibited in Spry mutant lenses, with concomitant ERK activation. Prox1 and p57(KIP2), normally upregulated at the onset of fiber differentiation were prematurely induced in the Spry mutant lens epithelial cells. However, terminal differentiation markers such as β- or γ-crystallin were not induced. Corneal epithelial precursors in Spry1 and -2 double mutants showed increased proliferation with elevated expression of Erm and DUSP6 and decreased expression of the corneal differentiation marker K12.
Conclusions: Collectively, the results indicate that Spry1 and -2 (1) through negative modulation of ERKs allow lens vesicle separation, (2) are targets of FGF signaling in the lens during initiation of fiber differentiation and (3) function redundantly in the corneal epithelial cells to suppress proliferation.
Figures



Similar articles
-
Spry1 and Spry2 are necessary for eyelid closure.Dev Biol. 2013 Nov 15;383(2):227-38. doi: 10.1016/j.ydbio.2013.09.014. Epub 2013 Sep 17. Dev Biol. 2013. PMID: 24055172 Free PMC article.
-
Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract.Exp Eye Res. 2016 Jan;142:92-101. doi: 10.1016/j.exer.2015.02.004. Epub 2015 May 21. Exp Eye Res. 2016. PMID: 26003864 Free PMC article. Review.
-
Activated Ras alters lens and corneal development through induction of distinct downstream targets.BMC Dev Biol. 2010 Jan 27;10:13. doi: 10.1186/1471-213X-10-13. BMC Dev Biol. 2010. PMID: 20105280 Free PMC article.
-
Sprouty is a negative regulator of transforming growth factor β-induced epithelial-to-mesenchymal transition and cataract.Mol Med. 2012 Jul 18;18(1):861-73. doi: 10.2119/molmed.2012.00111. Mol Med. 2012. PMID: 22517312 Free PMC article.
-
Cataract mutations and lens development.Prog Retin Eye Res. 1999 Mar;18(2):235-67. doi: 10.1016/s1350-9462(98)00018-4. Prog Retin Eye Res. 1999. PMID: 9932285 Review.
Cited by
-
Spry1 and Spry2 are necessary for eyelid closure.Dev Biol. 2013 Nov 15;383(2):227-38. doi: 10.1016/j.ydbio.2013.09.014. Epub 2013 Sep 17. Dev Biol. 2013. PMID: 24055172 Free PMC article.
-
Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract.Exp Eye Res. 2016 Jan;142:92-101. doi: 10.1016/j.exer.2015.02.004. Epub 2015 May 21. Exp Eye Res. 2016. PMID: 26003864 Free PMC article. Review.
-
Sprouty1 is a broad mediator of cellular senescence.Cell Death Dis. 2024 Apr 26;15(4):296. doi: 10.1038/s41419-024-06689-4. Cell Death Dis. 2024. PMID: 38670941 Free PMC article.
-
Heterogeneity in quiescent Müller glia in the uninjured zebrafish retina drive differential responses following photoreceptor ablation.Front Mol Neurosci. 2023 Jul 27;16:1087136. doi: 10.3389/fnmol.2023.1087136. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37575968 Free PMC article.
-
MicroRNA-124 facilitates lens epithelial cell apoptosis by inhibiting SPRY2 and MMP-2.Mol Med Rep. 2021 May;23(5):381. doi: 10.3892/mmr.2021.12020. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760112 Free PMC article.
References
-
- Coulombre JL, Coulombre AJ. Lens development: fiber elongation and lens orientation. Science. 1963;142:1489–1490 - PubMed
-
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377 - PubMed
-
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228 - PubMed
-
- Robinson ML, Ohtaka-Maruyama C, Chan CC, et al. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol. 1998;198:13–31 - PubMed
-
- Robinson ML, Overbeek PA, Verran DJ, et al. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

