The glia of Caenorhabditis elegans
- PMID: 21732423
- PMCID: PMC3117073
- DOI: 10.1002/glia.21084
The glia of Caenorhabditis elegans
Abstract
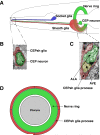
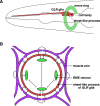
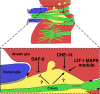
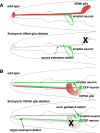
Glia have been, in many ways, the proverbial elephant in the room. Although glia are as numerous as neurons in vertebrate nervous systems, technical and other concerns had left research on these cells languishing, whereas research on neurons marched on. Importantly, model systems to study glia had lagged considerably behind. A concerted effort in recent years to develop the canonical invertebrate model animals, Drosophila melanogaster and Caenorhabditis elegans, as settings to understand glial roles in nervous system development and function has begun to bear fruit. In this review, we summarize our current understanding of glia and their roles in the nervous system of the nematode C. elegans. The recent studies we describe highlight the similarities and differences between C. elegans and vertebrate glia, and focus on novel insights that are likely to have general relevance to all nervous systems.
Copyright © 2010 Wiley-Liss, Inc.
Figures

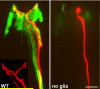

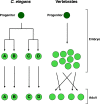
Similar articles
-
Glial development and function in the nervous system of Caenorhabditis elegans.Cold Spring Harb Perspect Biol. 2015 Jan 8;7(4):a020578. doi: 10.1101/cshperspect.a020578. Cold Spring Harb Perspect Biol. 2015. PMID: 25573712 Free PMC article. Review.
-
Glia actively sculpt sensory neurons by controlled phagocytosis to tune animal behavior.Elife. 2021 Mar 24;10:e63532. doi: 10.7554/eLife.63532. Elife. 2021. PMID: 33759761 Free PMC article.
-
Dye-filling of the amphid sheath glia: implications for the functional relationship between sensory neurons and glia in Caenorhabditis elegans.Biochem Biophys Res Commun. 2011 Mar 11;406(2):188-93. doi: 10.1016/j.bbrc.2011.02.003. Epub 2011 Feb 3. Biochem Biophys Res Commun. 2011. PMID: 21295547
-
Glia-Neuron Interactions in Caenorhabditis elegans.Annu Rev Neurosci. 2019 Jul 8;42:149-168. doi: 10.1146/annurev-neuro-070918-050314. Epub 2019 Mar 18. Annu Rev Neurosci. 2019. PMID: 30883261 Review.
-
Crosstalk between neurons and glia through G-protein coupled receptors: Insights from Caenorhabditis elegans.Prog Mol Biol Transl Sci. 2022;193(1):119-144. doi: 10.1016/bs.pmbts.2022.06.005. Epub 2022 Jul 22. Prog Mol Biol Transl Sci. 2022. PMID: 36357074
Cited by
-
Probing the enigma: unraveling glial cell biology in invertebrates.Curr Opin Neurobiol. 2013 Dec;23(6):1073-9. doi: 10.1016/j.conb.2013.07.002. Epub 2013 Jul 26. Curr Opin Neurobiol. 2013. PMID: 23896311 Free PMC article. Review.
-
The African turquoise killifish: A research organism to study vertebrate aging and diapause.Aging Cell. 2018 Jun;17(3):e12757. doi: 10.1111/acel.12757. Epub 2018 Mar 24. Aging Cell. 2018. PMID: 29573324 Free PMC article. Review.
-
Whole-Organism Developmental Expression Profiling Identifies RAB-28 as a Novel Ciliary GTPase Associated with the BBSome and Intraflagellar Transport.PLoS Genet. 2016 Dec 8;12(12):e1006469. doi: 10.1371/journal.pgen.1006469. eCollection 2016 Dec. PLoS Genet. 2016. PMID: 27930654 Free PMC article.
-
Ectopic fat deposition contributes to age-associated pathology in Caenorhabditis elegans.J Lipid Res. 2017 Jan;58(1):72-80. doi: 10.1194/jlr.M069385. Epub 2016 Nov 24. J Lipid Res. 2017. PMID: 27884963 Free PMC article.
-
The million-molecule challenge: a moonshot project to rapidly advance longevity intervention discovery.Geroscience. 2023 Dec;45(6):3103-3113. doi: 10.1007/s11357-023-00867-6. Epub 2023 Jul 11. Geroscience. 2023. PMID: 37432607 Free PMC article.
References
-
- Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol. 1981;198:435–451. - PubMed
-
- Albert PS, Riddle DL. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J Comp Neurol. 1983;219:461–481. - PubMed
-
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. - PubMed
-
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Filho WJ, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

