Human induced pluripotent cells resemble embryonic stem cells demonstrating enhanced levels of DNA repair and efficacy of nonhomologous end-joining
- PMID: 21718709
- PMCID: PMC3872739
- DOI: 10.1016/j.mrfmmm.2011.05.018
Human induced pluripotent cells resemble embryonic stem cells demonstrating enhanced levels of DNA repair and efficacy of nonhomologous end-joining
Abstract
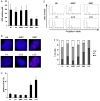
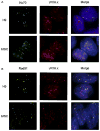
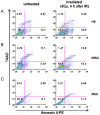
To maintain the integrity of the organism, embryonic stem cells (ESC) need to maintain their genomic integrity in response to DNA damage. DNA double strand breaks (DSBs) are one of the most lethal forms of DNA damage and can have disastrous consequences if not repaired correctly, leading to cell death, genomic instability and cancer. How human ESC (hESC) maintain genomic integrity in response to agents that cause DSBs is relatively unclear. Adult somatic cells can be induced to "dedifferentiate" into induced pluripotent stem cells (iPSC) and reprogram into cells of all three germ layers. Whether iPSC have reprogrammed the DNA damage response is a critical question in regenerative medicine. Here, we show that hESC demonstrate high levels of endogenous reactive oxygen species (ROS) which can contribute to DNA damage and may arise from high levels of metabolic activity. To potentially counter genomic instability caused by DNA damage, we find that hESC employ two strategies: First, these cells have enhanced levels of DNA repair proteins, including those involved in repair of DSBs, and they demonstrate elevated nonhomologous end-joining (NHEJ) activity and repair efficacy, one of the main pathways for repairing DSBs. Second, they are hypersensitive to DNA damaging agents, as evidenced by a high level of apoptosis upon irradiation. Importantly, iPSC, unlike the parent cells they are derived from, mimic hESC in their ROS levels, cell cycle profiles, repair protein expression and NHEJ repair efficacy, indicating reprogramming of the DNA repair pathways. Human iPSC however show a partial apoptotic response to irradiation, compared to hESC. We suggest that DNA damage responses may constitute important markers for the efficacy of iPSC reprogramming.
2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors indicate no potential conflict of interest.
Figures

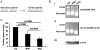

Similar articles
-
A human iPSC model of Ligase IV deficiency reveals an important role for NHEJ-mediated-DSB repair in the survival and genomic stability of induced pluripotent stem cells and emerging haematopoietic progenitors.Cell Death Differ. 2013 Aug;20(8):1089-100. doi: 10.1038/cdd.2013.44. Epub 2013 May 31. Cell Death Differ. 2013. PMID: 23722522 Free PMC article.
-
DNA double-strand break response in stem cells: mechanisms to maintain genomic integrity.Biochim Biophys Acta. 2013 Feb;1830(2):2345-53. doi: 10.1016/j.bbagen.2012.09.001. Epub 2012 Sep 17. Biochim Biophys Acta. 2013. PMID: 22995214 Review.
-
Error-prone nonhomologous end joining repair operates in human pluripotent stem cells during late G2.Aging (Albany NY). 2011 Jun;3(6):584-96. doi: 10.18632/aging.100336. Aging (Albany NY). 2011. PMID: 21685510 Free PMC article.
-
Mouse embryonic stem cells, but not somatic cells, predominantly use homologous recombination to repair double-strand DNA breaks.Stem Cells Dev. 2010 Nov;19(11):1699-711. doi: 10.1089/scd.2010.0058. Epub 2010 Aug 5. Stem Cells Dev. 2010. PMID: 20446816 Free PMC article.
-
DNA damage and repair in differentiation of stem cells and cells of connective cell lineages: A trigger or a complication?Eur J Histochem. 2021 May 3;65(2):3236. doi: 10.4081/ejh.2021.3236. Eur J Histochem. 2021. PMID: 33942598 Free PMC article. Review.
Cited by
-
Redox Homeostasis and Regulation in Pluripotent Stem Cells: Uniqueness or Versatility?Int J Mol Sci. 2021 Oct 11;22(20):10946. doi: 10.3390/ijms222010946. Int J Mol Sci. 2021. PMID: 34681606 Free PMC article. Review.
-
HiHiMap: single-cell quantitation of histones and histone posttranslational modifications across the cell cycle by high-throughput imaging.Mol Biol Cell. 2017 Aug 15;28(17):2290-2302. doi: 10.1091/mbc.E16-12-0870. Epub 2017 Jun 14. Mol Biol Cell. 2017. PMID: 28615324 Free PMC article.
-
DNA damage response in neonatal and adult stromal cells compared with induced pluripotent stem cells.Stem Cells Transl Med. 2015 Jun;4(6):576-89. doi: 10.5966/sctm.2014-0209. Epub 2015 Apr 21. Stem Cells Transl Med. 2015. PMID: 25900727 Free PMC article.
-
Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming.Cell Stem Cell. 2011 Oct 4;9(4):366-73. doi: 10.1016/j.stem.2011.07.018. Cell Stem Cell. 2011. PMID: 21982236 Free PMC article.
-
Oxygen as a Master Regulator of Human Pluripotent Stem Cell Function and Metabolism.J Pers Med. 2021 Sep 10;11(9):905. doi: 10.3390/jpm11090905. J Pers Med. 2021. PMID: 34575682 Free PMC article. Review.
References
-
- Bhattacharya B, Puri S, Puri RK. A review of gene expression profiling of human embryonic stem cell lines and their differentiated progeny. Curr Stem Cell Res Ther. 2009;4:98–106. - PubMed
-
- Rao RR, Stice SL. Gene expression profiling of embryonic stem cells leads to greater understanding of pluripotency and early developmental events. Biol Reprod. 2004;71:1772–1778. - PubMed
-
- Lengner CJ. iPS cell technology in regenerative medicine. Ann N Y Acad Sci. 2010;1192:38–44. - PubMed
-
- Carpenter MK, Couture LA. Regulatory considerations for the development of autologous induced pluripotent stem cell therapies. Regen Med. 2010;5:569–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

