CD28 expressed on malignant plasma cells induces a prosurvival and immunosuppressive microenvironment
- PMID: 21715687
- PMCID: PMC3152989
- DOI: 10.4049/jimmunol.1100016
CD28 expressed on malignant plasma cells induces a prosurvival and immunosuppressive microenvironment
Abstract
Interactions between the malignant plasma cells of multiple myeloma and stromal cells within the bone marrow microenvironment are essential for myeloma cell survival, mirroring the same dependence of normal bone marrow-resident long-lived plasma cells on specific marrow niches. These interactions directly transduce prosurvival signals to the myeloma cells and also induce niche production of supportive soluble factors. However, despite their central importance, the specific molecular and cellular components involved remain poorly characterized. We now report that the prototypic T cell costimulatory receptor CD28 is overexpressed on myeloma cells during disease progression and in the poor-prognosis subgroups and plays a previously unrecognized role as a two-way molecular bridge to support myeloid stromal cells in the microenvironment. Engagement by CD28 to its ligand CD80/CD86 on stromal dendritic cell directly transduces a prosurvival signal to myeloma cell, protecting it against chemotherapy and growth factor withdrawal-induced death. Simultaneously, CD28-mediated ligation of CD80/CD86 induces the stromal dendritic cell to produce the prosurvival cytokine IL-6 (involving novel cross-talk with the Notch pathway) and the immunosuppressive enzyme IDO. These findings identify CD28 and CD80/CD86 as important molecular components of the interaction between myeloma cells and the bone marrow microenvironment, point to similar interaction for normal plasma cells, and suggest novel therapeutic strategies to target malignant and pathogenic (e.g., in allergy and autoimmunity) plasma cells.
Conflict of interest statement
Figures
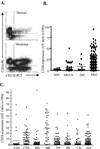

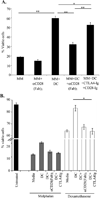
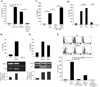
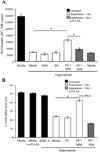
Similar articles
-
CD28-mediated regulation of multiple myeloma cell proliferation and survival.Blood. 2007 Jun 1;109(11):5002-10. doi: 10.1182/blood-2006-03-012542. Epub 2007 Feb 20. Blood. 2007. PMID: 17311991 Free PMC article.
-
CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma.Blood. 2014 Jun 12;123(24):3770-9. doi: 10.1182/blood-2013-10-530964. Epub 2014 Apr 29. Blood. 2014. PMID: 24782505 Free PMC article.
-
The Role of Marrow Microenvironment in the Growth and Development of Malignant Plasma Cells in Multiple Myeloma.Int J Mol Sci. 2021 Apr 24;22(9):4462. doi: 10.3390/ijms22094462. Int J Mol Sci. 2021. PMID: 33923357 Free PMC article. Review.
-
Osteoclast Immunosuppressive Effects in Multiple Myeloma: Role of Programmed Cell Death Ligand 1.Front Immunol. 2018 Aug 10;9:1822. doi: 10.3389/fimmu.2018.01822. eCollection 2018. Front Immunol. 2018. PMID: 30147691 Free PMC article. Review.
-
Malignant plasma cell lines express a functional CD28 molecule.Leukemia. 1998 Apr;12(4):610-8. doi: 10.1038/sj.leu.2400971. Leukemia. 1998. PMID: 9557621
Cited by
-
Inhibition of the aryl hydrocarbon receptor/polyamine biosynthesis axis suppresses multiple myeloma.J Clin Invest. 2018 Oct 1;128(10):4682-4696. doi: 10.1172/JCI70712. Epub 2018 Sep 10. J Clin Invest. 2018. PMID: 30198908 Free PMC article.
-
Therapeutic Potential of Innate Lymphoid Cells for Multiple Myeloma Therapy.Cancers (Basel). 2021 Sep 26;13(19):4806. doi: 10.3390/cancers13194806. Cancers (Basel). 2021. PMID: 34638291 Free PMC article. Review.
-
Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells.J Biol Chem. 2014 Mar 14;289(11):7747-62. doi: 10.1074/jbc.M113.519686. Epub 2014 Jan 10. J Biol Chem. 2014. PMID: 24415757 Free PMC article.
-
Experimental modeling of desensitization: What have we learned about preventing AMR?Am J Transplant. 2020 Jun;20 Suppl 4(Suppl 4):2-11. doi: 10.1111/ajt.15873. Am J Transplant. 2020. PMID: 32538533 Free PMC article. Review.
-
CD28-B7 interaction modulates short- and long-lived plasma cell function.J Immunol. 2012 Sep 15;189(6):2758-67. doi: 10.4049/jimmunol.1102728. Epub 2012 Aug 20. J Immunol. 2012. PMID: 22908331 Free PMC article.
References
-
- American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2010.
-
- Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–3720. - PubMed
-
- Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–4221. - PubMed
-
- Hurt EM, Wiestner A, Rosenwald A, Shaffer AL, Campo E, Grogan T, Bergsagel PL, Kuehl WM, Staudt LM. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer cell. 2004;5:191–199. - PubMed
-
- Jundt F, Probsting KS, Anagnostopoulos I, Muehlinghaus G, Chatterjee M, Mathas S, Bargou RC, Manz R, Stein H, Dorken B. Jagged1-induced Notch signaling drives proliferation of multiple myeloma cells. Blood. 2004;103:3511–3515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA121044/CA/NCI NIH HHS/United States
- R01 CA136671/CA/NCI NIH HHS/United States
- R01 CA136671-04/CA/NCI NIH HHS/United States
- R01 CA121044/CA/NCI NIH HHS/United States
- CA085183/CA/NCI NIH HHS/United States
- R01 CA133966/CA/NCI NIH HHS/United States
- R01 CA133966-04/CA/NCI NIH HHS/United States
- C06 RR020132/RR/NCRR NIH HHS/United States
- R01 AG020686-08/AG/NIA NIH HHS/United States
- R01 AG020686/AG/NIA NIH HHS/United States
- T32 CA085183/CA/NCI NIH HHS/United States
- R01 CA121044-05/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

