Autocatalytic activity of the ubiquitin-specific protease domain of herpes simplex virus 1 VP1-2
- PMID: 21715485
- PMCID: PMC3165783
- DOI: 10.1128/JVI.00798-11
Autocatalytic activity of the ubiquitin-specific protease domain of herpes simplex virus 1 VP1-2
Abstract
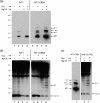
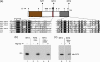
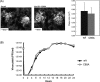
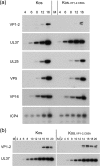
The herpes simplex virus (HSV) tegument protein VP1-2 is essential for virus entry and assembly. VP1-2 also contains a highly conserved ubiquitin-specific protease (USP) domain within its N-terminal region. Despite conservation of the USP and the demonstration that it can act on artificial substrates such as polyubiquitin chains, identification of the relevance of the USP in vivo to levels or function of any substrate remains limited. Here we show that HSV VP1-2 USP can act on itself and is important for stability. VP1-2 N-terminal variants encompassing the core USP domain itself were not affected by mutation of the catalytic cysteine residue (C65). However, extending the N-terminal region resulted in protein species requiring USP activity for accumulation. In this context, C65A mutation resulted in a drastic reduction in protein levels which could be stabilized by proteosomal inhibition or by the presence of normal C65. The functional USP domain could increase abundance of unstable variants, indicating action at least in part, in trans. Interestingly, full-length variants containing the inactive USP, although unstable when expressed in isolation, were stabilized by virus infection. The catalytically inactive VP1-2 retained complementation activity of a VP1-2-negative virus. Furthermore, a recombinant virus expressing a C65A mutant VP1-2 exhibited little difference in single-step growth curves and the kinetics and abundance of VP1-2 or a number of test proteins. Despite the absence of a phenotype for these replication parameters, the USP activity of VP1-2 may be required for function, including its own stability, under certain circumstances.
Figures




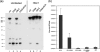
Similar articles
-
Functional analysis of nuclear localization signals in VP1-2 homologues from all herpesvirus subfamilies.J Virol. 2014 May;88(10):5391-405. doi: 10.1128/JVI.03797-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574406 Free PMC article.
-
Tsg101 interacts with herpes simplex virus 1 VP1/2 and is a substrate of VP1/2 ubiquitin-specific protease domain activity.J Virol. 2013 Jan;87(1):692-6. doi: 10.1128/JVI.01969-12. Epub 2012 Oct 17. J Virol. 2013. Retraction in: J Virol. 2013 Jun;87(11):6537. doi: 10.1128/JVI.00725-13. PMID: 23077308 Free PMC article. Retracted.
-
Characterization of the herpes simplex virus (HSV)-1 tegument protein VP1-2 during infection with the HSV temperature-sensitive mutant tsB7.J Gen Virol. 2009 Oct;90(Pt 10):2353-2363. doi: 10.1099/vir.0.012492-0. Epub 2009 Jul 8. J Gen Virol. 2009. PMID: 19587138
-
Identification of a highly conserved, functional nuclear localization signal within the N-terminal region of herpes simplex virus type 1 VP1-2 tegument protein.J Virol. 2008 Jun;82(11):5234-44. doi: 10.1128/JVI.02497-07. Epub 2008 Apr 2. J Virol. 2008. PMID: 18385239 Free PMC article.
-
A herpesvirus ubiquitin-specific protease is critical for efficient T cell lymphoma formation.Proc Natl Acad Sci U S A. 2007 Dec 11;104(50):20025-30. doi: 10.1073/pnas.0706295104. Epub 2007 Dec 4. Proc Natl Acad Sci U S A. 2007. PMID: 18056809 Free PMC article.
Cited by
-
Evasion of innate cytosolic DNA sensing by a gammaherpesvirus facilitates establishment of latent infection.J Immunol. 2015 Feb 15;194(4):1819-31. doi: 10.4049/jimmunol.1402495. Epub 2015 Jan 16. J Immunol. 2015. PMID: 25595793 Free PMC article.
-
A Nuclear localization signal in herpesvirus protein VP1-2 is essential for infection via capsid routing to the nuclear pore.J Virol. 2012 Sep;86(17):8998-9014. doi: 10.1128/JVI.01209-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718835 Free PMC article.
-
Viral Ubiquitin and Ubiquitin-Like Deconjugases-Swiss Army Knives for Infection.Biomolecules. 2020 Aug 1;10(8):1137. doi: 10.3390/biom10081137. Biomolecules. 2020. PMID: 32752270 Free PMC article. Review.
-
The HSV-1 ICP22 protein selectively impairs histone repositioning upon Pol II transcription downstream of genes.Nat Commun. 2023 Jul 31;14(1):4591. doi: 10.1038/s41467-023-40217-w. Nat Commun. 2023. PMID: 37524699 Free PMC article.
-
Functional analysis of nuclear localization signals in VP1-2 homologues from all herpesvirus subfamilies.J Virol. 2014 May;88(10):5391-405. doi: 10.1128/JVI.03797-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574406 Free PMC article.
References
-
- Bailey D., Barreca C., O'Hare P. 2007. Trafficking of the bZIP transmembrane transcription factor CREB-H into alternate pathways of ERAD and stress-regulated intramembrane proteolysis. Traffic 8:1796–1814 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

