Vaccination with dengue virus-like particles induces humoral and cellular immune responses in mice
- PMID: 21714940
- PMCID: PMC3144018
- DOI: 10.1186/1743-422X-8-333
Vaccination with dengue virus-like particles induces humoral and cellular immune responses in mice
Abstract
Background: The incidence of dengue, an infectious disease caused by dengue virus (DENV), has dramatically increased around the world in recent decades and is becoming a severe public health threat. However, there is currently no specific treatment for dengue fever, and licensed vaccine against dengue is not available. Vaccination with virus-like particles (VLPs) has shown considerable promise for many viral diseases, but the effect of DENV VLPs to induce specific immune responses has not been adequately investigated.
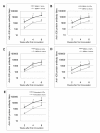
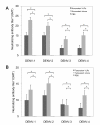
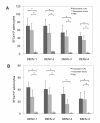
Results: By optimizing the expression plasmids, recombinant VLPs of four antigenically different DENV serotypes DENV1-4 were successfully produced in 293T cells. The vaccination effect of dengue VLPs in mice showed that monovalent VLPs of each serotype stimulated specific IgG responses and potent neutralizing antibodies against homotypic virus. Tetravalent VLPs efficiently enhanced specific IgG and neutralizing antibodies against all four serotypes of DENV. Moreover, vaccination with monovalent or tetravalent VLPs resulted in the induction of specific cytotoxic T cell responses.
Conclusions: Mammalian cell expressed dengue VLPs are capable to induce VLP-specific humoral and cellular immune responses in mice, and being a promising subunit vaccine candidate for prevention of dengue virus infection.
Figures

Similar articles
-
Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: immunological properties.BMC Microbiol. 2014 Dec 18;14:233. doi: 10.1186/s12866-014-0233-3. BMC Microbiol. 2014. PMID: 25520151 Free PMC article.
-
An Envelope-Modified Tetravalent Dengue Virus-Like-Particle Vaccine Has Implications for Flavivirus Vaccine Design.J Virol. 2017 Nov 14;91(23):e01181-17. doi: 10.1128/JVI.01181-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956764 Free PMC article.
-
Nanoparticle delivery of a tetravalent E protein subunit vaccine induces balanced, type-specific neutralizing antibodies to each dengue virus serotype.PLoS Negl Trop Dis. 2018 Sep 24;12(9):e0006793. doi: 10.1371/journal.pntd.0006793. eCollection 2018 Sep. PLoS Negl Trop Dis. 2018. PMID: 30248097 Free PMC article.
-
Preclinical and clinical development of a dengue recombinant subunit vaccine.Vaccine. 2015 Dec 10;33(50):7126-34. doi: 10.1016/j.vaccine.2015.09.101. Epub 2015 Oct 14. Vaccine. 2015. PMID: 26458804 Review.
-
Development of a recombinant, chimeric tetravalent dengue vaccine candidate.Vaccine. 2015 Dec 10;33(50):7112-20. doi: 10.1016/j.vaccine.2015.11.022. Epub 2015 Nov 14. Vaccine. 2015. PMID: 26585500 Review.
Cited by
-
COVID-19 vaccine platforms: Delivering on a promise?Hum Vaccin Immunother. 2021 Sep 2;17(9):2873-2893. doi: 10.1080/21645515.2021.1911204. Epub 2021 May 25. Hum Vaccin Immunother. 2021. PMID: 34033528 Free PMC article. Review.
-
Recent Advances in the Development of Virus-Like Particle-Based Flavivirus Vaccines.Vaccines (Basel). 2020 Aug 27;8(3):481. doi: 10.3390/vaccines8030481. Vaccines (Basel). 2020. PMID: 32867194 Free PMC article. Review.
-
Formation of Virus-Like Particles of the Dengue Virus Serotype 2 Expressed in Silkworm Larvae.Mol Biotechnol. 2019 Nov;61(11):852-859. doi: 10.1007/s12033-019-00210-5. Mol Biotechnol. 2019. PMID: 31473916
-
Use of virus-like particles and nanoparticle-based vaccines for combating picornavirus infections.Vet Res. 2024 Sep 30;55(1):128. doi: 10.1186/s13567-024-01383-x. Vet Res. 2024. PMID: 39350170 Free PMC article. Review.
-
Virus-like particle-induced protection against MRSA pneumonia is dependent on IL-13 and enhancement of phagocyte function.Am J Pathol. 2012 Jul;181(1):196-210. doi: 10.1016/j.ajpath.2012.03.018. Epub 2012 May 26. Am J Pathol. 2012. PMID: 22642909 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC) DoV-bID-DF. http://cdc.gov/ncidod/dvbid/dengue [cited]
-
- Sabchareon A, Lang J, Chanthavanich P. et al.Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am J Trop Med Hyg. 2002;66:264–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

