Effect of Src kinase phosphorylation on disordered C-terminal domain of N-methyl-D-aspartic acid (NMDA) receptor subunit GluN2B protein
- PMID: 21712388
- PMCID: PMC3191031
- DOI: 10.1074/jbc.M111.258897
Effect of Src kinase phosphorylation on disordered C-terminal domain of N-methyl-D-aspartic acid (NMDA) receptor subunit GluN2B protein
Abstract
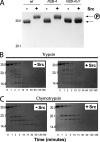
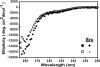
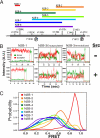
NMDA receptors are ligand-gated ion channels with a regulatory intracellular C-terminal domain (CTD). In GluN2B, the CTD is the largest domain in the protein but is intrinsically disordered. The GluN2B subunit is the major tyrosine-phosphorylated protein in synapses. Src kinase phosphorylates the GluN2B CTD, but it is unknown how this affects channel activity. In disordered proteins, phosphorylation can tip the balance between order and disorder. Transitions can occur in both directions, so it is not currently possible to predict the effects of phosphorylation. We used single molecule fluorescence to characterize the effects of Src phosphorylation on GluN2B. Scanning fluorescent labeling sites throughout the domain showed no positional dependence of the energy transfer. Instead, efficiency only scaled with the separation between labeling sites suggestive of a relatively featureless conformational energy landscape. Src phosphorylation led to a general expansion of the polypeptide, which would result in greater exposure of known protein-binding sites and increase the physical separation between contiguous sites. Phosphorylation makes the CTD more like a random coil leaving open the question of how Src exerts its effects on the NMDA receptor.
Figures
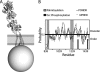

Similar articles
-
Tyrosine phosphorylation of the N-methyl-D-aspartate receptor by exogenous and postsynaptic density-associated Src-family kinases.J Neurochem. 2001 Aug;78(3):524-34. doi: 10.1046/j.1471-4159.2001.00433.x. J Neurochem. 2001. PMID: 11483655
-
Dopamine promotes NMDA receptor hypofunction in the retina through D1 receptor-mediated Csk activation, Src inhibition and decrease of GluN2B phosphorylation.Sci Rep. 2017 Jan 18;7:40912. doi: 10.1038/srep40912. Sci Rep. 2017. PMID: 28098256 Free PMC article.
-
NMDA channel regulation by channel-associated protein tyrosine kinase Src.Science. 1997 Jan 31;275(5300):674-8. doi: 10.1126/science.275.5300.674. Science. 1997. PMID: 9005855
-
Regulation of NMDA receptors by the tyrosine kinase Fyn.FEBS J. 2012 Jan;279(1):12-9. doi: 10.1111/j.1742-4658.2011.08391.x. Epub 2011 Dec 5. FEBS J. 2012. PMID: 21985328 Review.
-
The regulation of N-methyl-D-aspartate receptors by Src kinase.FEBS J. 2012 Jan;279(1):20-8. doi: 10.1111/j.1742-4658.2011.08413.x. Epub 2011 Dec 5. FEBS J. 2012. PMID: 22060915 Review.
Cited by
-
Regulation of the NMDA receptor by its cytoplasmic domains: (How) is the tail wagging the dog?Neuropharmacology. 2021 Sep 1;195:108634. doi: 10.1016/j.neuropharm.2021.108634. Epub 2021 Jun 20. Neuropharmacology. 2021. PMID: 34097949 Free PMC article. Review.
-
Behavioral and physiological characterization of PKC-dependent phosphorylation in the Grin2a∆PKC mouse.Brain Res. 2016 Sep 1;1646:315-326. doi: 10.1016/j.brainres.2016.06.022. Epub 2016 Jun 15. Brain Res. 2016. PMID: 27317637 Free PMC article.
-
Inhibition of SRC family kinases protects hippocampal neurons and improves cognitive function after traumatic brain injury.J Neurotrauma. 2014 Jul 15;31(14):1268-76. doi: 10.1089/neu.2013.3250. Epub 2014 Apr 17. J Neurotrauma. 2014. PMID: 24428562 Free PMC article.
-
Topography and motion of acid-sensing ion channel intracellular domains.Elife. 2021 Jul 22;10:e68955. doi: 10.7554/eLife.68955. Elife. 2021. PMID: 34292153 Free PMC article.
-
Excitatory and Mitogenic Signaling in Cell Death, Blood-brain Barrier Breakdown, and BBB Repair after Intracerebral Hemorrhage.Transl Stroke Res. 2012 Jul;3(Suppl 1):62-9. doi: 10.1007/s12975-012-0147-z. Epub 2012 Mar 15. Transl Stroke Res. 2012. PMID: 24323862
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

