Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression
- PMID: 21709185
- PMCID: PMC3157965
- DOI: 10.1200/JCO.2010.33.8038
Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression
Abstract
Purpose: This phase II trial was designed to assess the efficacy and safety of cetuximab, gemcitabine, and oxaliplatin followed by cetuximab, capecitabine, and radiation therapy in locally advanced pancreatic cancer (LAPC).
Patients and methods: Treatment-naive eligible patients (n = 69) received intravenous gemcitabine (1,000 mg/m(2)) and oxaliplatin (100 mg/m(2)) every 2 weeks for four doses, followed by radiation (50.4 Gy to the gross tumor only) with concurrent capecitabine (825 mg/m(2) twice daily on radiation treatment days). Cetuximab (500 mg/m(2)) was started on day 1 of chemotherapy and was continued every 2 weeks during chemotherapy and chemoradiotherapy. Diagnostic cytology specimens were immunostained for Smad4(Dpc4) expression.
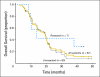
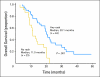
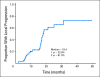
Results: Median overall survival time was 19.2 months (95% CI, 14.2 to 24.2 months), and 1-year, 2-year, and 4-year actuarial overall survival rates were 66.0%, 25.02%, and 11.3%, respectively. Acneiform rash correlated with improved survival (P = .001), but initial CA19-9, borderline resectable initial stage, and surgical resection (n = 7) did not. The 1-year and 2-year radiographic local progression rates were 22.8% and 61.0%, respectively. The worst acute toxic effects were GI toxicity (32% and 10% for grades 2 and 3, respectively); fatigue (26% and 6% for grades 2 and 3, respectively); sensory neuropathy (9% and 1% for grades 2 and 3, respectively); and acneiform rash (54% and 3% for grades 2 and 3, respectively). Smad4(Dpc4) expression correlated with a local rather than a distant dominant pattern of disease progression (P = .016).
Conclusion: This regimen appears effective and has acceptable toxicity. The primary end point (1-year overall survival rate > 45%) was met, with encouraging survival duration. Smad4(Dpc4) immunostaining correlated with the pattern of disease progression. Prospective validation of Smad4(Dpc4) expression in cytology specimens as a predictive biomarker is warranted and may lead to personalized treatment strategies for patients with localized pancreatic cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
Phase 2 trial of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer.Int J Radiat Oncol Biol Phys. 2014 Mar 15;88(4):837-44. doi: 10.1016/j.ijrobp.2013.12.030. Int J Radiat Oncol Biol Phys. 2014. PMID: 24606850 Free PMC article. Clinical Trial.
-
Phase II study of gemcitabine, oxaliplatin, and cetuximab in advanced pancreatic cancer.Am J Clin Oncol. 2012 Oct;35(5):446-50. doi: 10.1097/COC.0b013e31821862fb. Am J Clin Oncol. 2012. PMID: 21552097 Clinical Trial.
-
Induction Chemotherapy Followed by Concurrent Full-dose Gemcitabine and Intensity-modulated Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma.Am J Clin Oncol. 2016 Feb;39(1):1-7. doi: 10.1097/COC.0000000000000003. Am J Clin Oncol. 2016. PMID: 26132367
-
Cetuximab and chemoradiation for rectal cancer--is the water getting muddy?Acta Oncol. 2010 Apr;49(3):278-86. doi: 10.3109/02841860903536010. Acta Oncol. 2010. PMID: 20180626 Review.
-
Metastatic pancreatic cancer response to treatment with cetuximab and gemcitabine plus capecitabine: a case report and review of the literature.Tumori. 2010 Sep-Oct;96(5):764-7. doi: 10.1177/030089161009600520. Tumori. 2010. PMID: 21302625 Review.
Cited by
-
Krüppel-like Factor 10 as a Prognostic and Predictive Biomarker of Radiotherapy in Pancreatic Adenocarcinoma.Cancers (Basel). 2023 Oct 30;15(21):5212. doi: 10.3390/cancers15215212. Cancers (Basel). 2023. PMID: 37958386 Free PMC article. Review.
-
Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial.Lancet Oncol. 2013 Apr;14(4):317-26. doi: 10.1016/S1470-2045(13)70021-4. Epub 2013 Mar 6. Lancet Oncol. 2013. PMID: 23474363 Free PMC article. Clinical Trial.
-
Genomic profiling in pancreatic ductal adenocarcinoma and a pathway towards therapy individualization: A scoping review.Cancer Treat Rev. 2019 May;75:27-38. doi: 10.1016/j.ctrv.2019.03.003. Epub 2019 Mar 22. Cancer Treat Rev. 2019. PMID: 30927677 Free PMC article.
-
Metabolic Pathways as a Novel Landscape in Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2022 Aug 4;14(15):3799. doi: 10.3390/cancers14153799. Cancers (Basel). 2022. PMID: 35954462 Free PMC article. Review.
-
Biomarkers of TGF-β signaling pathway and prognosis of pancreatic cancer.PLoS One. 2014 Jan 20;9(1):e85942. doi: 10.1371/journal.pone.0085942. eCollection 2014. PLoS One. 2014. PMID: 24465802 Free PMC article.
References
-
- Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer: Definitive results of the 2000-2001 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. - PubMed
-
- Loehrer P, Powell M, Cardenes H, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol. 2008;26(suppl):214s. abstr 4506. - PubMed
-
- Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. - PubMed
-
- Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. - PubMed
-
- Xiong HQ, Rosenberg A, LoBuglio A, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II Trial. J Clin Oncol. 2004;22:2610–2616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

