Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice
- PMID: 21705482
- PMCID: PMC3165126
- DOI: 10.1093/carcin/bgr118
Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice
Abstract
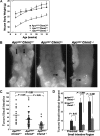
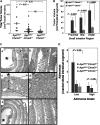
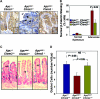
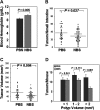
M3 subtype muscarinic receptors (CHRM3) are over-expressed in colon cancer. In this study, we used Apc(min/+) mice to identify the role of Chrm3 expression in a genetic model of intestinal neoplasia, explored the role of Chrm3 in intestinal mucosal development and determined the translational potential of inhibiting muscarinic receptor activation. We generated Chrm3-deficient Apc(min/+) mice and compared intestinal morphology and tumor number in 12-week-old Apc(min/+)Chrm3(-/-) and Apc(min/+)Chrm3(+/+) control mice. Compared with Apc(min/+)Chrm3(+/+) mice, Apc(min/+)Chrm3(-/-) mice showed a 70 and 81% reduction in tumor number and volume, respectively (P < 0.01). In adenomas, β-catenin nuclear staining was reduced in Apc(min/+)Chrm3(-/-) compared with Apc(min/+)Chrm3(+/+) mice (P < 0.02). Whereas Apc gene mutation increased the number of crypt and Paneth cells and decreased villus goblet cells, these changes were absent in Apc(min/+)Chrm3(-/-) mice. To determine whether pharmacological inhibition of muscarinic receptor activation attenuates intestinal neoplasia, we treated 6-week-old Apc(min/+) mice with scopolamine butylbromide, a non-subtype-selective muscarinic receptor antagonist. After 8 weeks of continuous treatment, scopolamine butylbromide-treated mice showed a 22% reduction in tumor number (P = 0.027) and a 36% reduction in tumor volume (P = 0.004) as compared with control mice. Compared with Chrm3 gene ablation, the muscarinic antagonist was less efficacious, most probably due to shorter duration of treatment and incomplete blockade of muscarinic receptors. Overall, these findings indicate that interplay of Chrm3 and β-catenin signaling is important for intestinal mucosal differentiation and neoplasia and provide a proof-of-concept that pharmacological inhibition of muscarinic receptor activation can attenuate intestinal neoplasia in vivo.
Figures
Similar articles
-
Scopolamine treatment and muscarinic receptor subtype-3 gene ablation augment azoxymethane-induced murine liver injury.J Pharmacol Exp Ther. 2010 Jun;333(3):639-49. doi: 10.1124/jpet.109.165118. Epub 2010 Mar 2. J Pharmacol Exp Ther. 2010. PMID: 20197374 Free PMC article.
-
Divergent effects of muscarinic receptor subtype gene ablation on murine colon tumorigenesis reveals association of M3R and zinc finger protein 277 expression in colon neoplasia.Mol Cancer. 2014 Apr 3;13:77. doi: 10.1186/1476-4598-13-77. Mol Cancer. 2014. PMID: 24694019 Free PMC article.
-
Sodium taurocholate inhibits intestinal adenoma formation in APCMin/+ mice, potentially through activation of the farnesoid X receptor.Carcinogenesis. 2010 Jun;31(6):1100-9. doi: 10.1093/carcin/bgq050. Epub 2010 Mar 1. Carcinogenesis. 2010. PMID: 20194350 Free PMC article.
-
Haploinsufficiency of Krüppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine.Cancer Res. 2009 May 15;69(10):4125-33. doi: 10.1158/0008-5472.CAN-08-4402. Epub 2009 May 12. Cancer Res. 2009. PMID: 19435907 Free PMC article.
-
Tensin4 (TNS4) is upregulated by Wnt signalling in adenomas in multiple intestinal neoplasia (Min) mice.Int J Exp Pathol. 2020 Jun;101(3-4):80-86. doi: 10.1111/iep.12352. Epub 2020 Jun 22. Int J Exp Pathol. 2020. PMID: 32567731 Free PMC article.
Cited by
-
A Critical Interpretive Synthesis of the Role of Arecoline in Oral Carcinogenesis: Is the Local Cholinergic Axis a Missing Link in Disease Pathophysiology?Pharmaceuticals (Basel). 2023 Dec 4;16(12):1684. doi: 10.3390/ph16121684. Pharmaceuticals (Basel). 2023. PMID: 38139811 Free PMC article. Review.
-
NF-κB Activator 1 downregulation in macrophages activates STAT3 to promote adenoma-adenocarcinoma transition and immunosuppression in colorectal cancer.BMC Med. 2023 Mar 29;21(1):115. doi: 10.1186/s12916-023-02791-0. BMC Med. 2023. PMID: 36978108 Free PMC article.
-
Modeling Intestinal Stem Cell Function with Organoids.Int J Mol Sci. 2021 Oct 9;22(20):10912. doi: 10.3390/ijms222010912. Int J Mol Sci. 2021. PMID: 34681571 Free PMC article. Review.
-
Muscarinic acetylcholine receptors: novel opportunities for drug development.Nat Rev Drug Discov. 2014 Jul;13(7):549-60. doi: 10.1038/nrd4295. Epub 2014 Jun 6. Nat Rev Drug Discov. 2014. PMID: 24903776 Free PMC article. Review.
-
Slc10a2-null mice uncover colon cancer-promoting actions of endogenous fecal bile acids.Carcinogenesis. 2015 Oct;36(10):1193-200. doi: 10.1093/carcin/bgv107. Epub 2015 Jul 25. Carcinogenesis. 2015. PMID: 26210740 Free PMC article.
References
-
- Frucht H, et al. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin. Cancer Res. 1999;5:2532–2539. - PubMed
-
- Yang WL, et al. Cholinergic receptor up-regulates COX-2 expression and prostaglandin E(2) production in colon cancer cells. Carcinogenesis. 2000;21:1789–1793. - PubMed
-
- Cheng K, et al. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003;63:6744–6750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

