High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study
- PMID: 21702021
- PMCID: PMC3498501
- DOI: 10.1002/art.30512
High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study
Abstract
Objective: Chronic infantile neurologic, cutaneous, articular (CINCA) syndrome, also known as neonatal-onset multisystem inflammatory disease (NOMID), is a dominantly inherited systemic autoinflammatory disease. Although heterozygous germline gain-of-function NLRP3 mutations are a known cause of this disease, conventional genetic analyses fail to detect disease-causing mutations in ∼40% of patients. Since somatic NLRP3 mosaicism has been detected in several mutation-negative NOMID/CINCA syndrome patients, we undertook this study to determine the precise contribution of somatic NLRP3 mosaicism to the etiology of NOMID/CINCA syndrome.
Methods: An international case-control study was performed to detect somatic NLRP3 mosaicism in NOMID/CINCA syndrome patients who had shown no mutation during conventional sequencing. Subcloning and sequencing of NLRP3 was performed in these mutation-negative NOMID/CINCA syndrome patients and their healthy relatives. Clinical features were analyzed to identify potential genotype-phenotype associations.
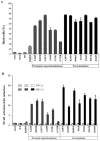
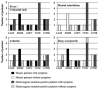
Results: Somatic NLRP3 mosaicism was identified in 18 of the 26 patients (69.2%). Estimates of the level of mosaicism ranged from 4.2% to 35.8% (mean ± SD 12.1 ± 7.9%). Mosaicism was not detected in any of the 19 healthy relatives (18 of 26 patients versus 0 of 19 relatives; P < 0.0001). In vitro functional assays indicated that the detected somatic NLRP3 mutations had disease-causing functional effects. No differences in NLRP3 mosaicism were detected between different cell lineages. Among nondescript clinical features, a lower incidence of mental retardation was noted in patients with somatic mosaicism. Genotype-matched comparison confirmed that patients with somatic NLRP3 mosaicism presented with milder neurologic symptoms.
Conclusion: Somatic NLRP3 mutations were identified in 69.2% of patients with mutation-negative NOMID/CINCA syndrome. This indicates that somatic NLRP3 mosaicism is a major cause of NOMID/CINCA syndrome.
Copyright © 2011 by the American College of Rheumatology.
Figures
Similar articles
-
A somatic NLRP3 mutation as a cause of a sporadic case of chronic infantile neurologic, cutaneous, articular syndrome/neonatal-onset multisystem inflammatory disease: Novel evidence of the role of low-level mosaicism as the pathophysiologic mechanism underlying mendelian inherited diseases.Arthritis Rheum. 2010 Apr;62(4):1158-66. doi: 10.1002/art.27342. Arthritis Rheum. 2010. PMID: 20131270
-
Somatic NLRP3 mosaicism in Muckle-Wells syndrome. A genetic mechanism shared by different phenotypes of cryopyrin-associated periodic syndromes.Ann Rheum Dis. 2015 Mar;74(3):603-10. doi: 10.1136/annrheumdis-2013-204361. Epub 2013 Dec 10. Ann Rheum Dis. 2015. PMID: 24326009
-
Brief Report: whole-exome sequencing revealing somatic NLRP3 mosaicism in a patient with chronic infantile neurologic, cutaneous, articular syndrome.Arthritis Rheumatol. 2014 Jan;66(1):197-202. doi: 10.1002/art.38217. Arthritis Rheumatol. 2014. PMID: 24431285 Free PMC article.
-
Diagnosis of cryopyrin-associated periodic syndrome: challenges, recommendations and emerging concepts.Expert Rev Clin Immunol. 2015;11(7):827-35. doi: 10.1586/1744666X.2015.1047765. Epub 2015 May 15. Expert Rev Clin Immunol. 2015. PMID: 25979514 Review.
-
Current status of understanding the pathogenesis and management of patients with NOMID/CINCA.Curr Rheumatol Rep. 2011 Apr;13(2):123-31. doi: 10.1007/s11926-011-0165-y. Curr Rheumatol Rep. 2011. PMID: 21538043 Free PMC article. Review.
Cited by
-
Genetic Mosaicism as a Cause of Inborn Errors of Immunity.J Clin Immunol. 2021 May;41(4):718-728. doi: 10.1007/s10875-021-01037-z. Epub 2021 Apr 16. J Clin Immunol. 2021. PMID: 33864184 Free PMC article. Review.
-
Identification of Interleukin-1β-Producing Monocytes That Are Susceptible to Pyronecrotic Cell Death in Patients With Neonatal-Onset Multisystem Inflammatory Disease.Arthritis Rheumatol. 2015 Dec;67(12):3286-97. doi: 10.1002/art.39307. Arthritis Rheumatol. 2015. PMID: 26245468 Free PMC article.
-
Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes.Arthritis Rheum. 2012 Jul;64(7):2375-86. doi: 10.1002/art.34409. Arthritis Rheum. 2012. PMID: 22294344 Free PMC article. Clinical Trial.
-
Characterization of NLRP3 variants in Japanese cryopyrin-associated periodic syndrome patients.J Clin Immunol. 2012 Apr;32(2):221-9. doi: 10.1007/s10875-011-9629-0. Epub 2011 Dec 24. J Clin Immunol. 2012. PMID: 22193915
-
Pathophysiology, clinical manifestations and current management of IL-1 mediated monogenic systemic autoinflammatory diseases, a literature review.Pediatr Rheumatol Online J. 2022 Oct 17;20(1):90. doi: 10.1186/s12969-022-00728-0. Pediatr Rheumatol Online J. 2022. PMID: 36253853 Free PMC article. Review.
References
-
- Neven B, Callebaut I, Prieur AM, Feldmann J, Bodemer C, Lepore L, et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood. 2004;103:2809–15. - PubMed
-
- Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17:586–99. - PubMed
-
- Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. - PubMed
-
- Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

