Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission
- PMID: 21701560
- PMCID: PMC3160255
- DOI: 10.1038/emboj.2011.198
Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission
Abstract
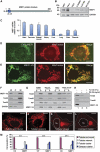
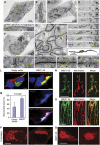
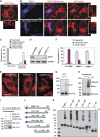
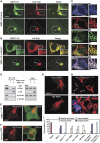
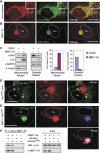
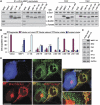
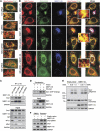
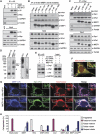
Mitochondrial morphology is controlled by two opposing processes: fusion and fission. Drp1 (dynamin-related protein 1) and hFis1 are two key players of mitochondrial fission, but how Drp1 is recruited to mitochondria and how Drp1-mediated mitochondrial fission is regulated in mammals is poorly understood. Here, we identify the vertebrate-specific protein MIEF1 (mitochondrial elongation factor 1; independently identified as MiD51), which is anchored to the outer mitochondrial membrane. Elevated MIEF1 levels induce extensive mitochondrial fusion, whereas depletion of MIEF1 causes mitochondrial fragmentation. MIEF1 interacts with and recruits Drp1 to mitochondria in a manner independent of hFis1, Mff (mitochondrial fission factor) and Mfn2 (mitofusin 2), but inhibits Drp1 activity, thus executing a negative effect on mitochondrial fission. MIEF1 also interacts with hFis1 and elevated hFis1 levels partially reverse the MIEF1-induced fusion phenotype. In addition to inhibiting Drp1, MIEF1 also actively promotes fusion, but in a manner distinct from mitofusins. In conclusion, our findings uncover a novel mechanism which controls the mitochondrial fusion-fission machinery in vertebrates. As MIEF1 is vertebrate-specific, these data also reveal important differences between yeast and vertebrates in the regulation of mitochondrial dynamics.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Comment in
-
How to split up: lessons from mitochondria.EMBO J. 2011 Jul 20;30(14):2751-3. doi: 10.1038/emboj.2011.219. EMBO J. 2011. PMID: 21772324 Free PMC article.
-
Membrane dynamics: MIEF1 mingles with mitochondria.Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):464. doi: 10.1038/nrm3161. Nat Rev Mol Cell Biol. 2011. PMID: 21779019 No abstract available.
Similar articles
-
The mitochondrial elongation factors MIEF1 and MIEF2 exert partially distinct functions in mitochondrial dynamics.Exp Cell Res. 2013 Nov 1;319(18):2893-904. doi: 10.1016/j.yexcr.2013.07.010. Epub 2013 Jul 20. Exp Cell Res. 2013. PMID: 23880462
-
Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy.Autophagy. 2019 Dec;15(12):2107-2125. doi: 10.1080/15548627.2019.1596494. Epub 2019 Mar 28. Autophagy. 2019. PMID: 30894073 Free PMC article.
-
Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission.J Biol Chem. 2013 Sep 20;288(38):27584-27593. doi: 10.1074/jbc.M113.479873. Epub 2013 Aug 6. J Biol Chem. 2013. PMID: 23921378 Free PMC article.
-
The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease.Clin Sci (Lond). 2016 Nov 1;130(21):1861-74. doi: 10.1042/CS20160030. Clin Sci (Lond). 2016. PMID: 27660309 Review.
-
New insights into the function and regulation of mitochondrial fission.Biochim Biophys Acta. 2013 May;1833(5):1256-68. doi: 10.1016/j.bbamcr.2013.02.002. Epub 2013 Feb 20. Biochim Biophys Acta. 2013. PMID: 23434681 Review.
Cited by
-
FUNDC1 regulates mitochondrial dynamics at the ER-mitochondrial contact site under hypoxic conditions.EMBO J. 2016 Jul 1;35(13):1368-84. doi: 10.15252/embj.201593102. Epub 2016 May 4. EMBO J. 2016. PMID: 27145933 Free PMC article.
-
MTCH2 cooperates with MFN2 and lysophosphatidic acid synthesis to sustain mitochondrial fusion.EMBO Rep. 2024 Jan;25(1):45-67. doi: 10.1038/s44319-023-00009-1. Epub 2023 Dec 14. EMBO Rep. 2024. PMID: 38177900 Free PMC article.
-
Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission.Mol Biol Cell. 2013 Mar;24(5):659-67. doi: 10.1091/mbc.E12-10-0721. Epub 2013 Jan 2. Mol Biol Cell. 2013. PMID: 23283981 Free PMC article.
-
TRAP1 controls mitochondrial fusion/fission balance through Drp1 and Mff expression.PLoS One. 2012;7(12):e51912. doi: 10.1371/journal.pone.0051912. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284813 Free PMC article.
-
march5 Governs the Convergence and Extension Movement for Organization of the Telencephalon and Diencephalon in Zebrafish Embryos.Mol Cells. 2020 Jan 31;43(1):76-85. doi: 10.14348/molcells.2019.0210. Mol Cells. 2020. PMID: 31910335 Free PMC article.
References
-
- Chan DC (2006) Mitochondrial fusion and fission in mammals. Ann Rev Cell Dev Biol 22: 79–99 - PubMed
-
- Chang CR, Blackstone C (2007) Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587 - PubMed
-
- Chen H, Chomyn A, Chan DC (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

